Figures & data
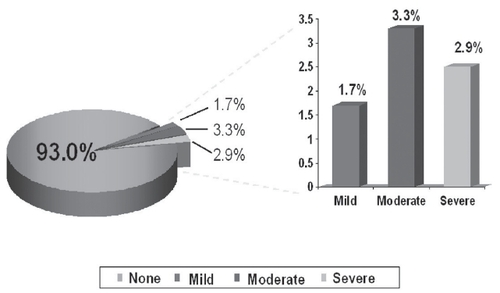
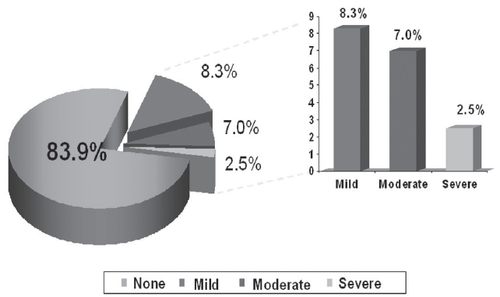
Figure 3 Dry mouth tolerability in subjects treated with transdermal oxybutynin versus immediate-release oxybutynin.
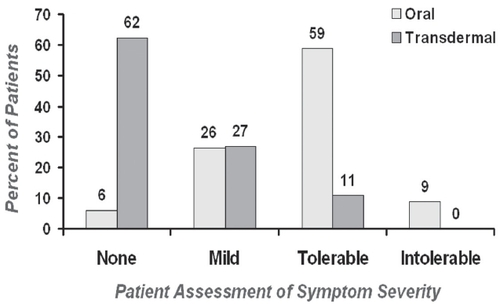
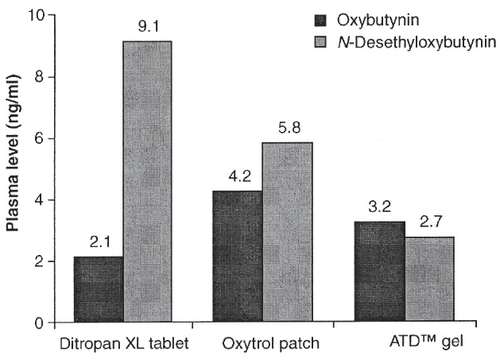
Figure 6 Oxybutynin and DEO levels based on route of administration. Copyright © 2005. Alberti I, Grenier A, Kraus H, et al. 2005. Pharmaceutical development and clinical effectiveness of a novel gel technology for transdermal drug delivery. Expert Opin Drug Deliv, 2:935–50.