Figures & data
Figure 1 Erlotinib hydrochloride molecule: N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine; C22H23N3O4.HCl; MW 429.90.
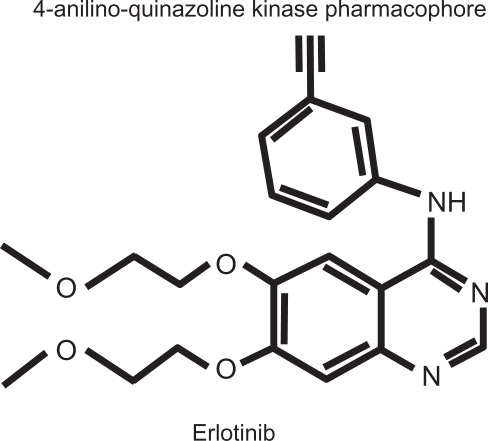
Figure 2 Design of the phase III trial of erlotinib in first-line advanced NSCLC with EGFR mutations in Europe: the EURTACC trial.Citation54
Abbreviations: ECOG, Eastern Oncology Cooperative Group; EGFR, epidermal growth factor receptor; NSCLC, non-small-cell lung cancer; ORR, objective response rate; PD, progressive disease; QoL, quality of life.
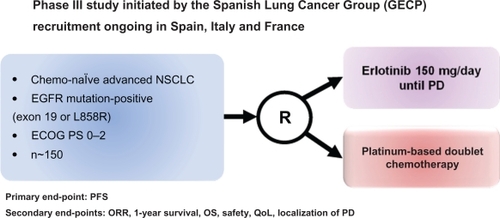
Figure 3 Design of the phase III trial of erlotinib in first line advanced NSCLC with EGFR mutations in Asian population: the OPTIMAL trial.Citation55
Abbreviations: ECOG, Eastern Oncology Cooperative Group; EGFR, epidermal growth factor receptor; NSCLC, non-small-cell lung cancer; PD, progressive disease; PFS, progression-free survival; ORR, overall response rate; OS, overall survival; QoL, quality of life.
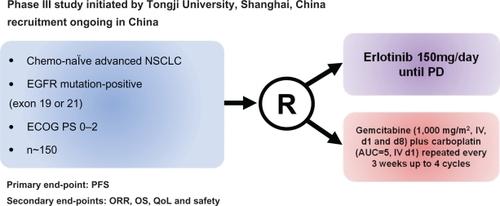
Figure 4 Current strategies to treat advanced NSCLC patients.
Abbreviations: NSCLC, non-small-cell lung cancer; PD, progressive disease; CR, complete response; PR, partial response; SD, stable disease.
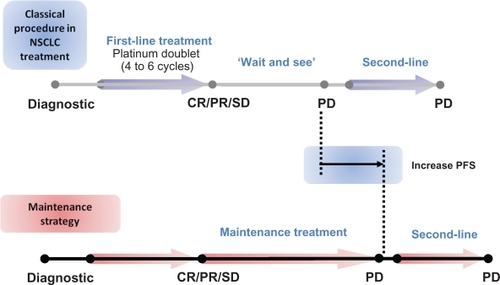
Table 1 Randomized trials of maintenance therapy with erlotinib
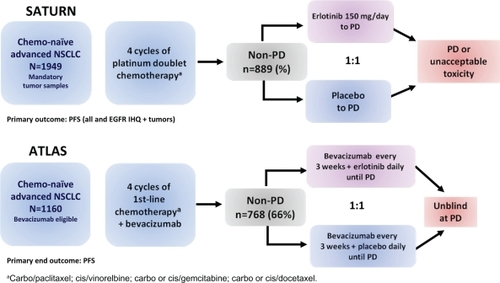
Figure 5 Design of erlotinib maintenance phase III trials in advanced NSCLC treatment (SATURNCitation73 and ATLASCitation74).
Abbreviations: NSCLC, non-small cell lung cancer; non-PD, complete responses, partial responses, stable disease; PD, progressive disease; PFS, progression-free survival.