Figures & data
Figure 1 Mode of action of GnRH receptor antagonists.Citation58 Reproduced with permission from Anderson J. Degarelix: a novel gonadotropin-releasing hormone blocker for the treatment of prostate cancer. Future Oncol. 2009;5(4):433–443.Citation58 Copyright © 2009 Future Medicine Ltd.
Abbreviations: GnRH, gonadotrophin-releasing hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
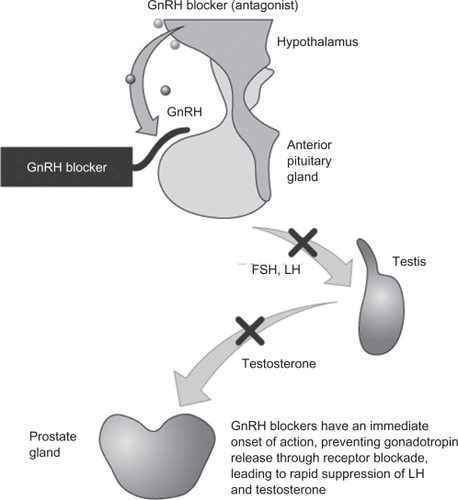
Figure 2 Mean (n = 8; ± SEM) testosterone levels in the intact rat induced by degarelix, abarelix, azaline B, and ganirelix, administered at a dose of 2 mg/kg in 5% mannitol, compared with surgical castration. Reproduced with permission from Broqua P, Riviere PJ, Conn PM, Rivier JE, Aubert ML, Junien JL. Pharmacological profile of a new, potent, and long-acting gonadotropin-releasing hormone antagonist: degarelix. J Pharmacol Exp Ther. 2002;301:95–102.Citation25 Copyright © 2002 American Society for Pharmacology & Experimental Therapeutics.
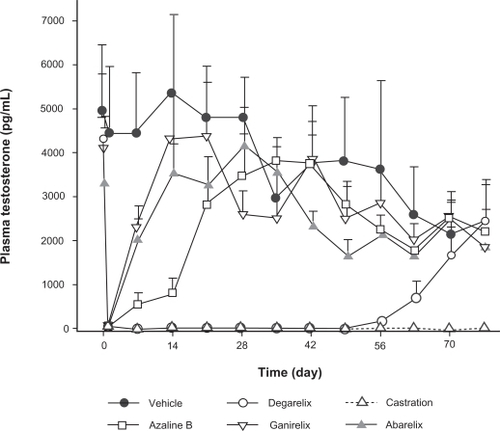
Figure 3 Reconstitution time distribution profiles for degarelix doses of 80 mg (Panel A) and 120 mg (Panel B) (three batches combined).
Table 1 Percentage of patients with serum testosterone levels ≤0.5 ng/mL (responders) during monthly measurements from day 28 through to day 364 in phase II and III degarelix studiesCitation36–Citation38,Citation58
Figure 4 Median testosterone levels with degarelix and leuprolide. Panel A depicts the first month of treatment; Panel B shows data from across the 1-year treatment period.Citation38 Reproduced with permission from Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102(11):1531–1538.Citation38 Copyright © 2008 Blackwell Publishing Ltd.
*P < 0.001 degarelix (both doses) versus leuprolide.
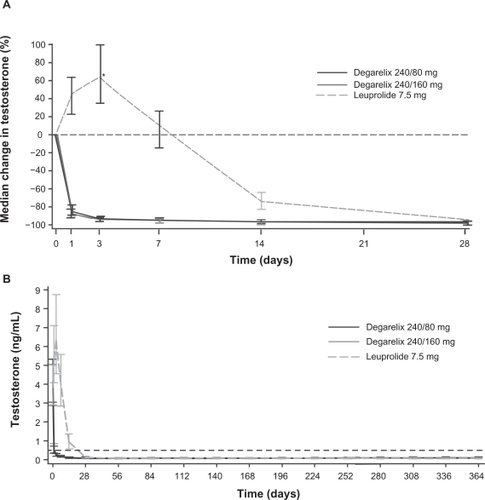
Figure 5 Median percentage change from baseline in PSA levels with degarelix and leuprolide. Panel A depicts the first month of treatment; Panel B shows data from across the 1-year treatment period. Reproduced with permission from Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102(11):1531–1538.Citation38 Copyright © 2008 Blackwell Publishing Ltd.
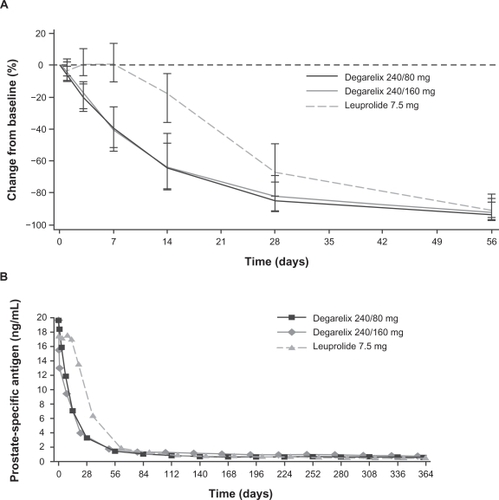
Table 2 Incidence and intensity of adverse events during degarelix and leuprolide treatment (incidence of ≥5% in any group)