Figures & data
Figure 1 Example of a chromatogram of the HPLC-determination of fluticasone-17-propionate in a diluted sample of the admixture of Flutide® forte Fertiginhalat “ready to use” 2,0 mg/2 ml with 2,0 ml Atrovent® LS and 0,5 ml Sultanol® after 5 h storage at room temperature.
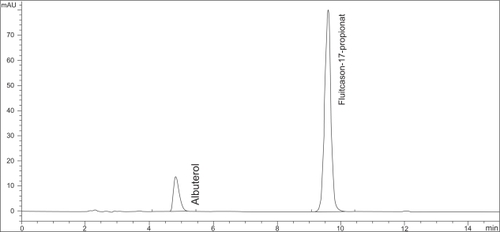
Figure 2 Example of a chromatogram of the simultaneous HPLC-determination of ipratopium and albuterol in a 1:10 diluted sample of the admixture of Flutide® forte “ready to use” 2.0 mg/2 ml with 2.0 ml Atrovent® LS and 0.5 ml Sultanol® after 5 h storage at room temperature.
The peak at retention time ~ 1 minute is designated to excipients in the nebulizable drugs.
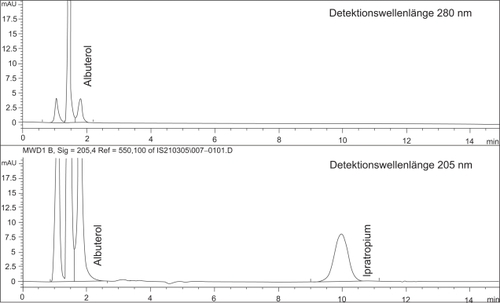
Table 4 Osmolality and pH values of the pure nebulizer suspension/solutions Flutide® forte “ready to use”, Atrovent® LS and Sultanol® Inhalationslösung (Inhalation solution) and mixtures of these 3 nebulizable drugs, stored under ambient light conditions at room temperature