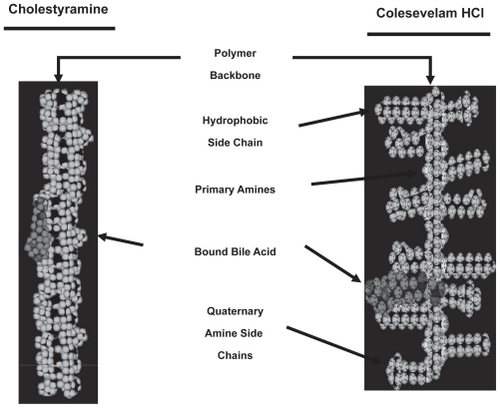
Figures & data
Figure 1 The enterohepatic circulation of bile acids.
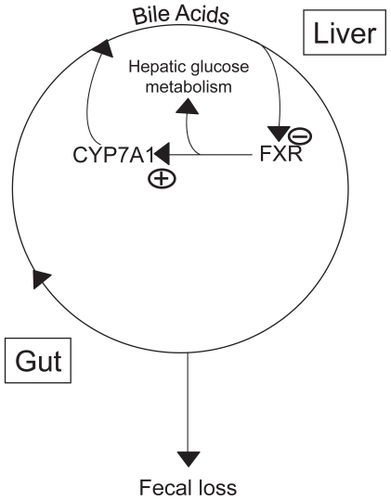
Figure 3 Percentage change in total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride (TG) levels from baseline (week 0) to the end of treatment (week 6) in patients who received placebo or colesevelam hydrochloride therapy. Reproduced with permission from Davidson MH, Dillon MA, Gordon B, et al Colesevelam hydrochloride (Cholestagel): a new, potent bile acid sequestrant associated with a low incidence of gastrointestinal. side effects. Arch Intern Med. 1999;159:1893–1900.Citation32 Copyright © 1999 American Medical Association. All rights reserved.
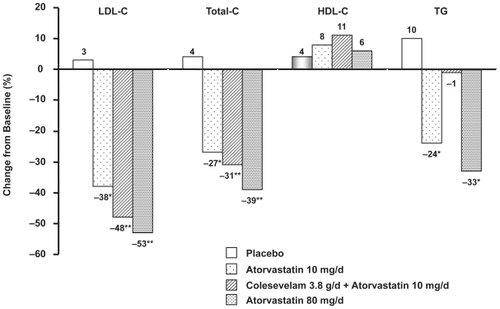
Figure 4 Effect of addition of colesevelam to atorvastatin 10 mg. LDL-C and Total-C values are expressed as mean; HDL-C and TG values are expressed as median; *p < 0.05 vs placebo **p < 0.05 vs atorvastatin 10 mg. Reproduced with permission from Hunninghake D. Insull W Jr., Toth P. et al, Coadministration of colesevelam hydrochloride with atorvastatin lowers LDL cholesterol additively.] Atherosclerosis. 2001;158:407–416.Citation36 Copyright © 2001 Elsevier.
![Figure 4 Effect of addition of colesevelam to atorvastatin 10 mg. LDL-C and Total-C values are expressed as mean; HDL-C and TG values are expressed as median; *p < 0.05 vs placebo **p < 0.05 vs atorvastatin 10 mg. Reproduced with permission from Hunninghake D. Insull W Jr., Toth P. et al, Coadministration of colesevelam hydrochloride with atorvastatin lowers LDL cholesterol additively.] Atherosclerosis. 2001;158:407–416.Citation36 Copyright © 2001 Elsevier.](/cms/asset/22937c9a-cf86-4080-9f20-f5e3938622da/dmso_a_3866_f0004_b.jpg)
Figure 5 Summary of effect of add-on colesevelam 3.75 g/day to metformin-, sulfonylurea-, and insulin-based treatment on the placebo-controlled HbA1c reduction at study end from baseline, in subjects with type 2 diabetes (intention to treat population, last observation carried forward).Citation41,Citation50,Citation51
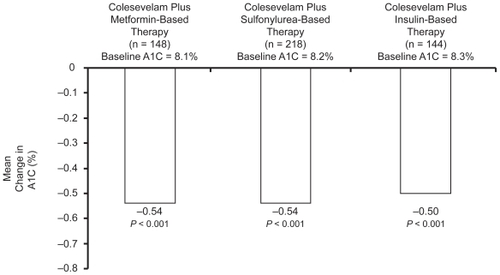
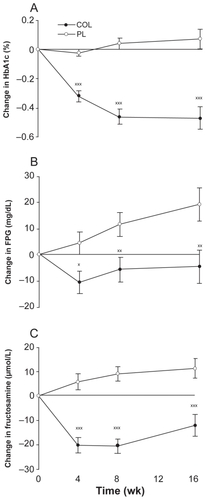
Figure 6 Least squares mean (SEM) change from baseline in glycated hemoglobin A1c (HbA1c) A), fasting plasma glucose (FPG) B), and fructosamine C) levels in subjects (intent-to-treat population without last observation carried forward imputation) receiving colesevelam hydrochloride, 3.75 g/d, or placebo for 16 weeks. Goldberg RB, Fonseca VA, Truitt KE, Jones MR. Efficacy and safety of colesevelam in patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-based therapy. Arch Intern Med. 2008;168(14):1531–1540.Citation41 Copyright © 2008 American Medical Association. All rights reserved.