Figures & data
Figure 1 Overview of the clinical study program of saxagliptin leading to approval.Citation45
Abbreviations: MET, metformin; OL, open-label; SU, sulfonylurea; T2DM, type 2 diabetes mellitus; TZD, thiazolidinedione.
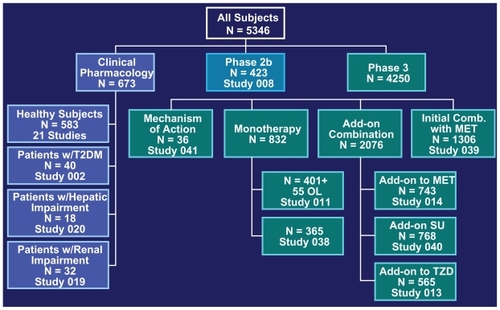
Figure 2 Pooled data of the HbA1c reductions observed in the clinical studies with saxagliptin (SAXA):Citation34–Citation36,Citation38–Citation40,Citation45 The left hand side of the figure shows the pooled monotherapy results, the right side shows the HbA1c reductions in various combinations with either metformin (MET), thiazolidinedione (TZD) or sulfonylurea (SU). In each study, the results for doses with 2.5 mg saxagliptin od (light bars) or 5 mg saxagliptin od (dark bars) are shown.
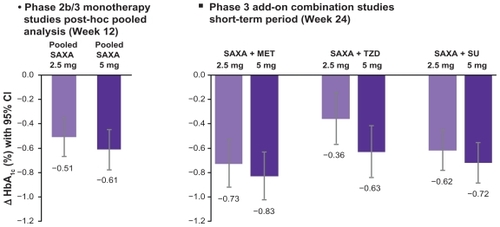
Table 1 Monotherapy studies with saxagliptin
Table 2 Add on combination therapy studies with saxagliptin
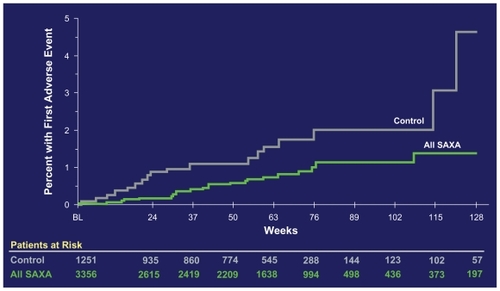
Figure 3 Cardiovascular safety data of saxagliptin (SAXA). The cumulative incidence of major adverse cardiovascular events (MACE) is shown.Citation43 MACE is defined as a combined “major adverse cardiovascular event” consisting of the items cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. On the ordinate of the figure, the percentage of patients having had a first incident of the MACE-defining adverse events are depicted.