Figures & data
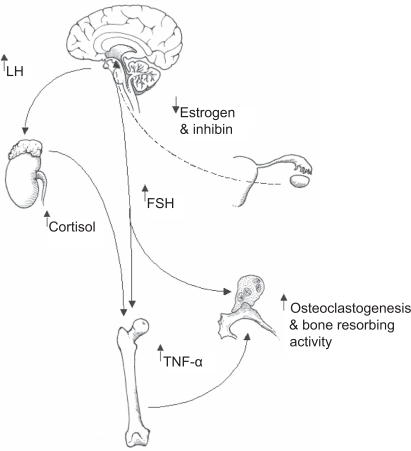
Figure 1 A summary of how follicle stimulating hormone (FSH) and luteinizing hormone (LH) might co-operate in promoting bone catabolism postmenopause. Elevated levels of LH are known to induce adrenal cortex hyperfunction and resultant increases in circulating cortisol. It is postulated that increased cortisol in response to raised LH postmenopause impacts upon bone forming osteoblasts by inhibiting bone matrix synthesis through dampening osteoblast activity. In the long term the effect of raised cortisol will contribute to osteopaenia and the subsequent development of osteoporosis. Increasing levels of FSH could target at least two different compartments of bone tissue; the bone resorbing osteoclast population and bone marrow macrophage precursors. With regard to the former, FSH has been shown to directly activate osteoclastic resorption. With reference to the bone marrow, resident macrophage precursors are now known to mobilise tumor necrosis factor alpha (TNF-α) in response to FSH. TNF-α is a key player in the demise of bone postmenopause through stimulating osteoclastic bone resorption. In this model elevated LH and FSH co-operate in fuelling net bone loss thereby increasing the risk of developing osteoporosis.