Figures & data
Figure 1 The copolymer-mediated sequestering and integration of multiple therapeutics into a single thin film is shown. Langmuir-Blodgett was used to deposit drug containing triblock copolymer films that can maintain drug incorporation for localized release. Combinatorial therapy at the surface of the material–tissue interface can provide significantly enhanced treatment produced by a noninvasive nanoscale platform. Copyright © 2008. Adapted from CitationPierstorff E, Krucoff M, Ho D. 2008. Apoptosis induction and attenuation of inflammatory gene expression in murine macrophages via multitherapeutic nanomembranes. Nanotechnology, 19:265103–12.
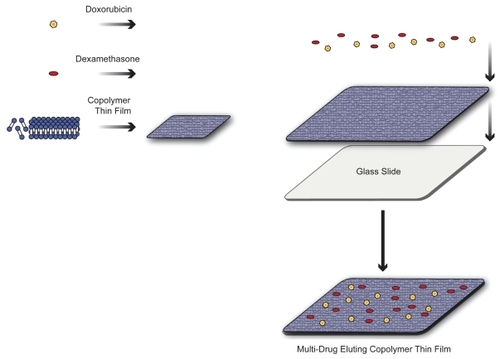
Figure 2 Confirmation of doxorubicin hydrochloride and dexamethasone drug incorporation into the PMOXA–PDMS–PMOXA triblock copolymer was performed via monitoring of surface pressure changes of fabricated Langmuir films. Subsequent drug activity trials confirmed the functionality of deposited hybrid material.
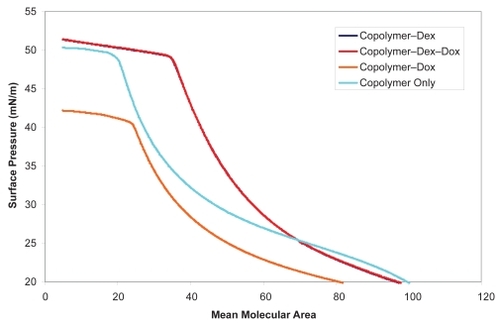
Figure 3 A) RT-PCR analysis of IL-6 gene expression was done following macrophage incubation with the polymer–Dex–Dox nanofilms. Gene expression assays with aqueous Dex added were conducted as a control for Dex activity. Results indicated the incorporation of Dex into the copolymer–Dex–Dox thin film and a potent drug releasing activity as shown through a substantial reduction in inflammatory gene expression. B) In addition, RT-PCR analysis of TNFα gene expression was done following macrophage incubation with the polymer–Dex–Dox nanofilms. Gene expression assays with aqueous Dex added were conducted as a control for Dex activity. Results further indicated the incorporation of Dex into the copolymer–Dex–Dox thin film and a potent drug releasing activity as shown through a substantial reduction in inflammatory gene expression. The broad suppression of inflammatory gene expression demonstrates an efficient hybrid nanofilm system for an active drug delivery interface. Data was acquired and responses were observed from a minimum of three trials.
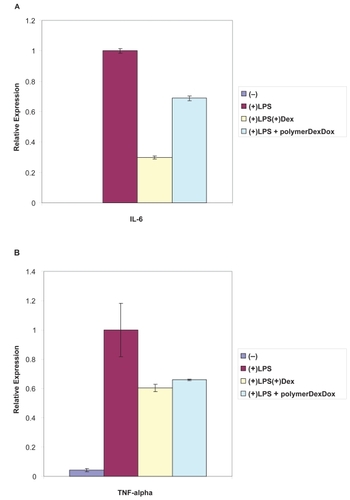
Figure 4 DNA fragmentation assays revealed the preserved functionality of Dox in polymer–Dex–Dox nanofilms. Lane 1 is a DNA marker. Lane 2 represents the control without polymer or drug. Lane 3 represents the culturing of macrophages atop polymer only substrates. Lane 4 represents the polymer–Dox hybrid activity as shown through the clear fragmentation of DNA. Lane 5 represents the polymer–Dex–Dox hybrid activity as shown through the clear fragmentation of DNA. Controlled and localized chemotherapeutic release would significantly enhance the efficacy of cancer treatment via the reduction of systemic toxicity due to systemic administration. The lack of DNA fragmentation or cell death of cells grown on the copolymer substrate highlight the biocompatible properties of the copolymer.
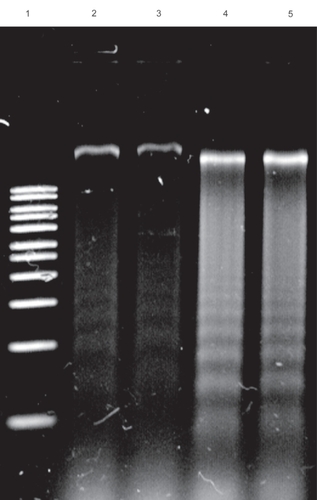
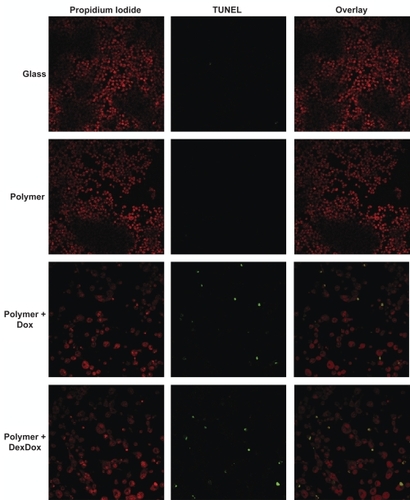
Figure 5 Microscopy Dox impact upon cellular proliferation and morphology was demonstrated via confocal microscopy. Macrophages cultured atop glass or polymer only substrates produced highly confluent growth. Macrophages cultured atop polymer–Dox and polymer–Dex–Dox nanofilm hybrids generated decrease cell confluency, enlarged, preapoptotic cells, as well as shrunken apoptotic cells due to Dox-induced cell death/apoptosis. TUNEL assays demonstrate the progression of cells grown on polymer–Dox and polymer–Dex–Dox nanofilm hybrids to apoptosis.
Notes: Magnification: 40X, Leica inverted microscope Confocal Laser Scanning System.