Figures & data
Figure 1 Summary of patient flow throughout study.
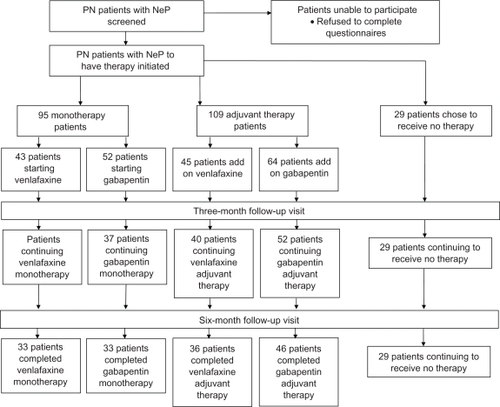
Table 1 Clinical features and baseline characteristics of patients and control subjects studied
Table 2 Parameters measured for monotherapy groups at baseline, 3 and 6 months after initiation of treatment
Table 3 Parameters measured for adjuvant groups at baseline, 3 and 6 months after initiation of treatment
Table 4 Parameters measured for the control groups at baseline, 3 and 6 months after initiation of treatment
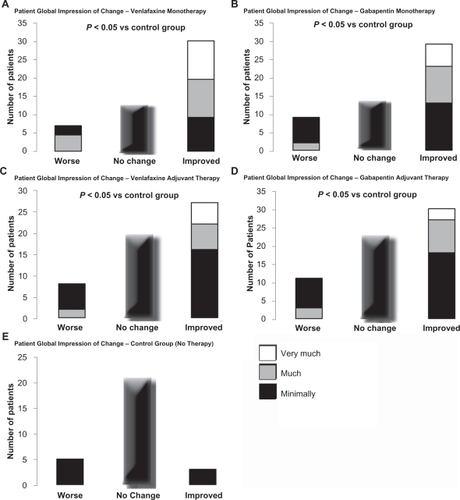
Figure 2 Patient global impression of change (PGIC) was analyzed using a Cochran-Mantel-Haenszel procedure, adjusting for center in each case. Patients reported a significant perceived benefit with monotherapy compared to control group patients for each of venlafaxine (A) and gabapentin (B), as well as with adjuvant therapy for each of venlafaxine (C) and gabapentin (D). In contrast, the control group receiving no therapy had no significant change in PGIC reported (E).