Figures & data
Figure 1 APP proteolytic processing and major fates of the amyloid β (Aβ) fragment. Sequential cleavage by β-secretase (BACE-1) followed by γ-secretase [containing Presenilin 1 and 2 (PS1/2)] generates Aβ. This fragment has several fates. (a) It can aggregate and accumulate as extraneuronal plaques which characterize Alzheimer’s dementia. (b) It can be cleared from the extracellular space (a mechanism that may be altered in AD brains). (c) It can be degraded by a variety of proteases such as Insulin Degrading Enzyme (IDE), Neprilyisn (NEP), Plasmin, Plasminogen Activator (uPA/tPA), Endothelin Enzyme-1 or Matrix Metalloprotease-9 (MMP-9). Hypothetically, at least, it might return to the plasma membrane and insert in oligomer form as an ion channel. γ-secretase cleavage is also thought to liberate an intracellular domain (AICD) which may influence gene transcription.
![Figure 1 APP proteolytic processing and major fates of the amyloid β (Aβ) fragment. Sequential cleavage by β-secretase (BACE-1) followed by γ-secretase [containing Presenilin 1 and 2 (PS1/2)] generates Aβ. This fragment has several fates. (a) It can aggregate and accumulate as extraneuronal plaques which characterize Alzheimer’s dementia. (b) It can be cleared from the extracellular space (a mechanism that may be altered in AD brains). (c) It can be degraded by a variety of proteases such as Insulin Degrading Enzyme (IDE), Neprilyisn (NEP), Plasmin, Plasminogen Activator (uPA/tPA), Endothelin Enzyme-1 or Matrix Metalloprotease-9 (MMP-9). Hypothetically, at least, it might return to the plasma membrane and insert in oligomer form as an ion channel. γ-secretase cleavage is also thought to liberate an intracellular domain (AICD) which may influence gene transcription.](/cms/asset/c0d5347e-fc48-4e56-ab0b-60aa2289b5d5/dndt_a_12160207_f0001_b.jpg)
Figure 2 Sequential cleavage by β-secretase and γ-secretase releases Aβ fragment of varying length. β-secretase cleavage has to occur following internalization of APP-enzyme complex at pH values around 4.0 in cytosolic locations.
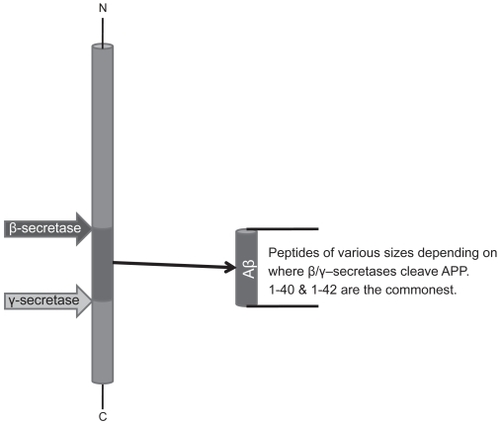
Figure 3 APP internalization and generation of Aβ. APP is trafficked through constitutive secretory pathways, undergoes post-translational modification and ultimately locates to the plasma membrane. Poorly understood mechanisms/signals then effect internalization/endocytosis of APP to intracellular sites where optimum pH exists for activation of is β-secretase (BACE-1) that process APP.
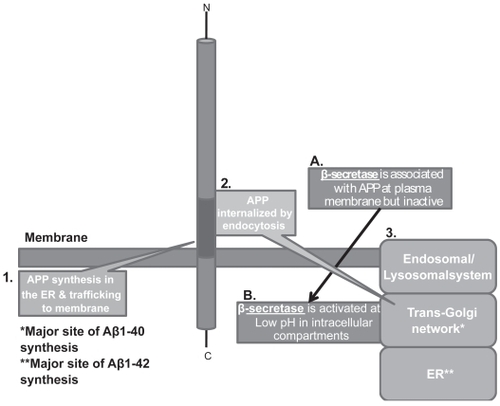
Table 1 Summary of native neuronal ion channels
Table 2 Summary of selected key publications which support, directly or indirectly, the ion channel hypothesis of AD pathogenesis. Note relative dominance of in vitro systems and that the majority of studies utilized exogenous Amyloid β1–40