Figures & data
Figure 1 Patients reporting pain relief at time points up to 2 hours following dosing with rizatriptan or sumatriptan in a randomized, double-blind, placebo-controlled, cross-over study (CitationGoldstein et al 1998). Adults with at least a 6-month history of migraine with or without aura were randomized to treat 2 sequential migraine attacks of moderate to severe intensity separated by at least 5 days. Treatment sequences included (a) rizatriptan 5 mg followed by sumatriptan 25 mg or vice versa, (b) rizatriptan 10 mg followed by sumatriptan 50 mg or vice versa, or placebo followed by placebo (data not shown). Hazard ratios for time to pain relief indicate that patients receiving rizatriptan were significantly more likely to achieve pain relief during the 2-hour period than patients receiving sumatriptan. *p < 0.05. Reproduced from CitationGoldstein J, Ryan R, Jiang K, et al. 1998. Crossover comparison of rizatriptan 5 mg and 10 mg vs sumatriptan 25 mg and 50 mg in migraine. Rizatriptan Protocol 046 Study Group. Headache, 38:737–47. Copyright © 1998, with permission from Blackwell Publishing Ltd.
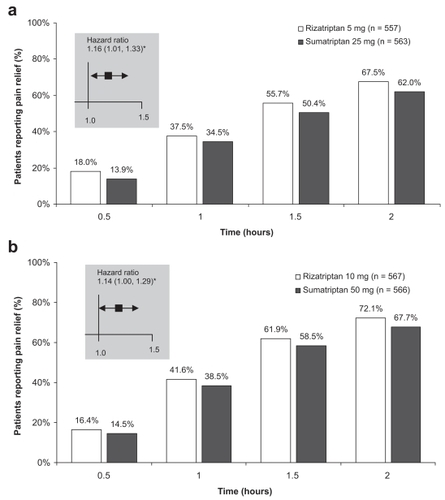
Table 1 Comparative efficacy of rizatriptan in the acute treatment of migraine in randomized, double-blind, comparative studies
Table 2 Efficacy of rizatriptan in the acute treatment of migraine in retrospective analyses of comparative studies
Figure 2 Median percent of migraine attacks in which patients achieved pain relief or pain-free status 2 hours after dosing. (a) Results in patients enrolled in an open-label, randomized, 6-month extension study who treated at least 1 moderate or severe migraine attack with rizatriptan 5-mg wafers (median of 16 attacks treated), rizatriptan 10-mg wafers (median 13 attacks treated), or standard care (primarily sumatriptan) (median 14 attacks treated). (b) Results in patients enrolled in a 12-month, randomized extension study who treated at least 1 migraine with rizatriptan 5-mg tablets (median of 14 attacks treated), rizatriptan 10-mg tablets (median 21 attacks treated), or standard care (primarily nonsteroidal anti-inflammatory drugs) (median 19 attacks treated). *p < 0.05 vs rizatriptan 5 mg and vs standard care. reproduced from CitationCady R, Crawford G, Ahrens S, et al. 2001. Long-term efficacy and tolerability of rizatriptan wafers in migraine. Medscape General Medicine, 3(4): http://www.medscape.com/viewarticle/408137 © 2001 Medscape; reproduced from CitationBlock GA, Goldstein J, Polis A, et al. 1998. Efficacy and safety of rizatriptan vs standard care during long-term treatment for migraine. Rizatriptan Multicenter Study Groups. Headache, 38:764–71. Copyright © 1998, with permission from Blackwell Publishing Ltd.
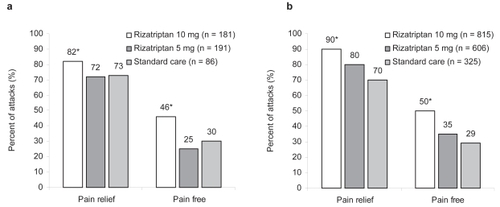
Table 3 Clinical summary of rizatriptan in the treatment of migraine