Figures & data
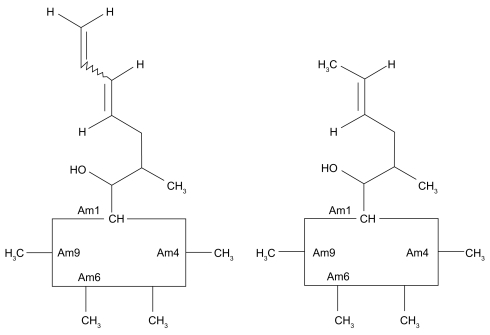
Figure 2 Comparison of calcineurin inhibition of voclosporin versus CsA.
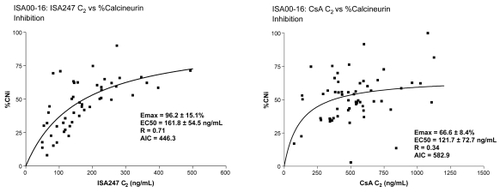
Figure 3 Emax versus reduction in PASI in phase 3 psoriasis study.
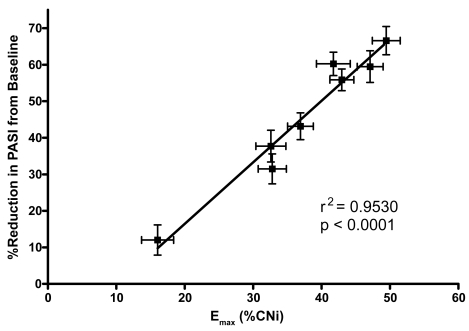
Figure 4 Voclosporin therapeutic window for renal toxicity.
Table 1 Selected inclusion and exclusion criteria for the LUMINATE clinical trials program (Protocol 1, p. 22–24; Protocol 2, p. 22–25; Protocol 3, p. 20–23)
Table 2 Primary, secondary, and additional endpoints of the LUMINATE clinical trial programTable Footnote* (Protocol 1, p. 18; Protocol 2, p. 18; Protocol 3, p. 17)