Figures & data
Figure 1 Eye muscle and orbital fibroblast autoantigens recognized by T lymphocytes or antibodies in thyroid-associated ophthalmopathy (TAO). Our working hypothesis is that the ocular myopathy subtype of TAO is initiated by T lymphocyte-mediated targeting of calsequestrin or a yet unidentified eye muscle cell membrane antigen. Serum antibodies against flavoprotein, G2s and sarcalumenin are likely to be secondary to release of the proteins following muscle fiber necrosis. Chronic eyelid disease, which is a feature of TAO or a dominant sign in patients with Hashimoto’s thyroiditis, may be the result of T lymphocyte-mediated targeting of calsequestrin in the upper eyelid levator palpebrae superioris muscle. The congestive ophthalmopathy subtype of TAO is likely to result from a reaction against the TSH-r or collagen XIII in the fibroblast cell membranes, which leads to fibroblast stimulation and excess production of collagen and glycosaminoglycans. The different reactions shown in the figure may occur alone or in combination. Reproduced with the permission from Tani J, Wall JR. Autoimmunity against eye-muscle antigens may explain thyroid-associated ophthalmopathy. CMAJ. 2006;175(3):239.Citation10 Copyright © 2006 Canadian Medical Association
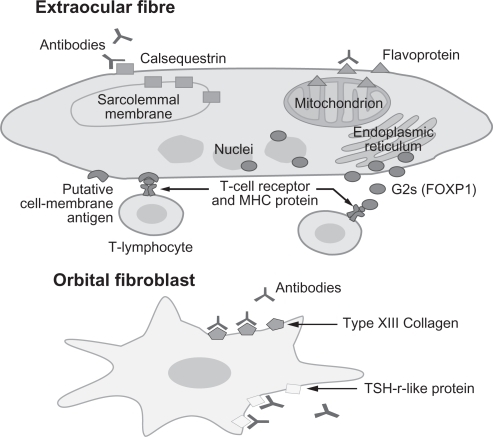
Figure 2 Orbital fibroblast activation in thyroid-associated ophthalmopathy. The main features of Graves’ ophthalmopathy include the enlargement of eye muscles and the expansion of the orbital fatty connective tissues. These changes result from accumulation of glycosaminoglycans (GAGs) and edema within these tissues and de novo adipogenesis. The orbital fibroblast (OF) appears to be the major effector cell in GO influencing both processes. In the active stage of disease, OF are capable of producing large amounts of GAGs following (i) stimulation by cytokines (especially Th-1 cytokines), (ii) activation of CD40 by CD40 ligand and (iii) activation of insulin growth factor receptor (IGF-1R) by autoantibodies. A subset of OF, the preadipocytes, are capable of differentiating into mature adipocytes following appropriate stimulation by cytokines (IL-6) and activation of thyroid-stimulating hormone receptor (TSH-r). In addition, OF have been shown to display the immunoregulatory molecules major histocompatibility complex MHC class II (HLA-DR) and intercellular adhesion molecule-1 (ICAM-1), and also are capable of secreting chemokines and cytokines which stimulate the infiltration of activated T cells into areas of inflammation. In the ‘burn-out’ stage of ophthalmopathy, OF participate in tissue fibrosis. Reproduced with permission from Bednarczuk T, Gopinath B, Ploski R, Wall JR. Susceptibility genes in Graves’ ophthalmopathy: searching for a needle in a haystack? Clin Endocrinol (Oxf). 2007; 67(1):3–19.Citation45 Copyright © 2007 Wiley Blackwell.
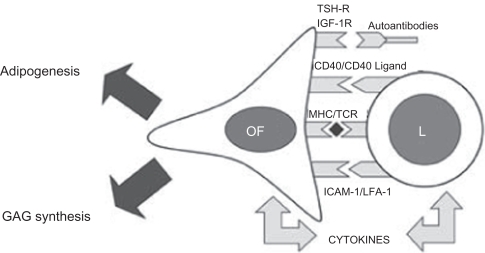
Table 1 Thyroid associated ophthalmology (TAO) subtypes, clinical features and candidate autoantibodies