Figures & data
Figure 1 UV radiation induced apoptosis is enhanced by wortmannin’s inhibition of PI3K related kinases. MCF-7 (A) and Saos2 (C) cells exposed to 50 or 100 J/m2 UV for 24, 48, or 72 hours were analyzed by Western blotting. MCF-7 (B) and Saos2 (D) cells were grown in the presence or absence of wortmannin, irradiated as above and analyzed for DNA content after 24 and 48 hours. The percentage of cells with hypodiploid DNA content is indicated.
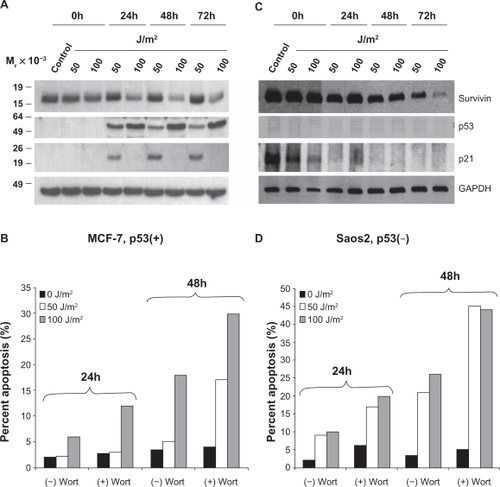
Figure 2 UV radiation’s repartitioning of survivin from the cytosol to the mitochondria and nucleus is reduced after wortmannin treatment in p53 expressing MCF-7 cells. MCF-7 and Saos2 cells, exposed to 50 or 100 J/m2 UV for 24 hours were subcellularly fractionated into cytosol (A), mitochondria (B) and nuclear (C) fractions and analyzed by Western blotting.
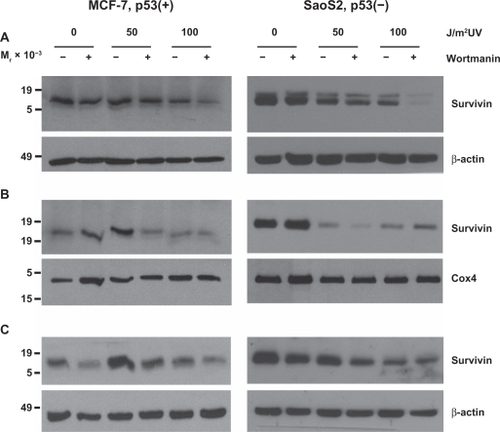
Figure 3 Loss of DNA-PK, ATM and expression of a kinase dead (kd) ATR renders cells sensitive to UV radiation. M059K (DNA-PK+/+), M059J (DNA-PK−/−), GM02184E (ATM+/+), GM03189D (ATM−/−), GM847 (ATR+/+) and GM847Kd (ATR kinase dead) were exposed to 50 or 100 J/m2 UV and analyzed for DNA content after (A) 24 and (B) 48 hours. The percentage of cells with hypodiploid DNA content is indicated.
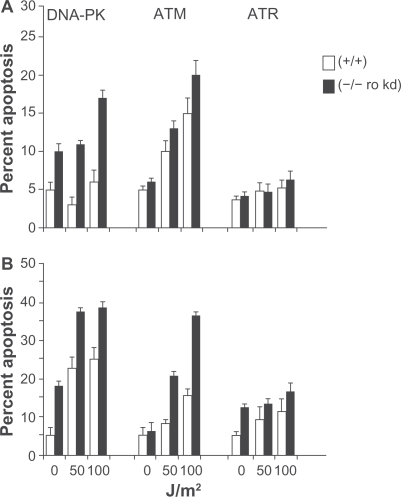
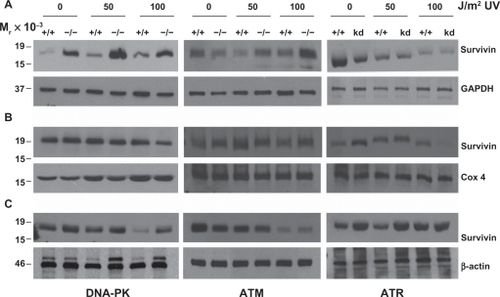
Figure 4 Survivin, p53 and p21, after UV radiation, are differentially expressed in PI3K related kinase competent versus incompetent cells. A) M059K (DNA-PK+/+), M059J (DNA-PK−/−), B) GM02184E (ATM+/+), GM03189D (ATM−/−), C) GM847 (ATR+/+) and GM847Kd (ATR kinase dead) were exposed to 50 or 100 J/m2 UV and analyzed by Western blotting after 24, 48, and 72 hours.
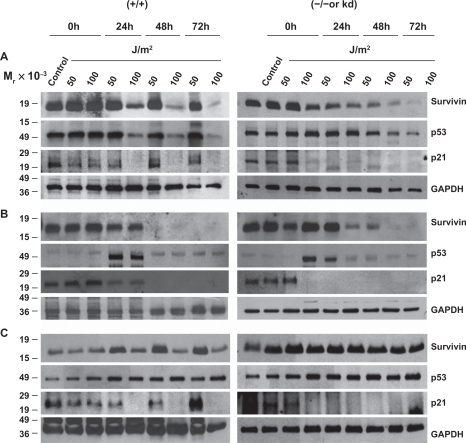
Figure 5 UV radiation repartitions survivin from the cytosol to the mitochondria and nucleus. M059K (DNA-PK+/+), M059J (DNA-PK−/−), GM02184E (ATM+/+), GM03189D (ATM−/−), GM847 (ATR+/+) and GM847Kd (ATR kinase dead), exposed to 50 or 100 J/m2 UV for 24 hours were subcellularly fractionated into cytosol (A), mitochondria (B) and nuclear (C) fractions and analyzed by Western blotting.
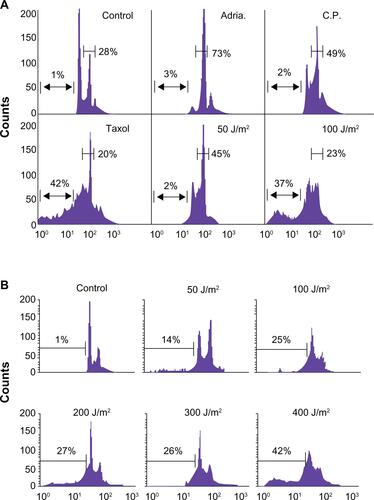
Supplemental Figure 1 (A) Low dose genotoxic stressors such as Adriamycin (Adria., 100 nM), Cisplatin (C.P., 3 mM) and UV (50 J/m2) arrest MCF-7 cells in the G2/M phase of the cell cycle while high-dose UV (doses ≥ 100 J/m2) or the tubulin poison Taxol (2 mM) induce cellular death. (B) Breast carcinoma MCF-7 cells were irradiated with 50–400 J/m2 UV under minimal 1X PBS. PBS was immediately removed and complete culture medium was added. After 24 hours, cells were harvested and analyzed for DNA content by propidium iodide staining and flow cytometry. Percentages of apoptotic cells with hypodiploid (sub-G1) DNA content are indicated per each condition tested. Data are representative of one of two independent experiments with comparable results.