Figures & data
Table 1 WHO classification of tumors subsumed under the category “Malignant glioma”
Figure 1 Simplified diagram of the two major pathways affected by EGFR-mediated signaling in malignant glioma cells. The PI3 kinase pathway is shown on the right and the Ras/Map kinase pathway on the left. Following EGFR activation, a series of sequential phosphorylation events result in cell proliferation and survival through increased transcription and translation of key proteins while inhibiting pro-apoptotic pathways. Agents that target key points in the pathway that are discussed in the text are shown in parentheses at their respective sites of action.
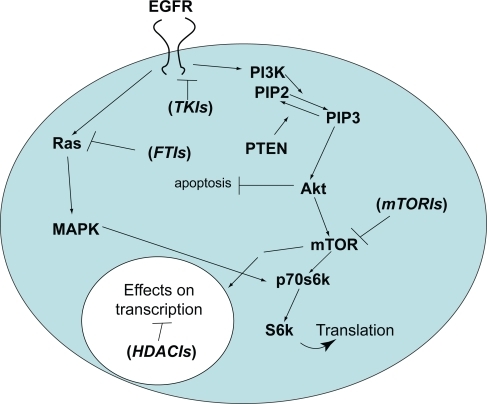
Figure 2 MRI scans showing a typical prolonged response to single-agent erlotinib in a patient with progressive GBM. The image in the left panel is consistent with a partially necrotic enhancing mass and surrounding peri-tumoral edema. The image on the right, obtained 16 months after the initiation of erlotinib, reveals a decrease in the mass and associated edema. The patient was on high-dose dexamethasone treatment (to control cerebral edema) at the time of erlotinib treatment initiation and was off all dexamethasone at the time of the follow-up scan.
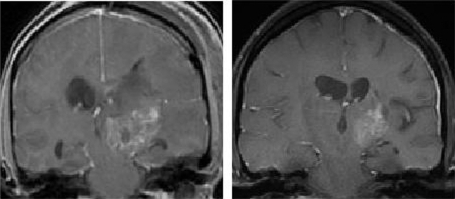
Table 2 Epidermal growth factor receptor tyrosine kinase inhibitor trials in adult malignant gliomas
Figure 3 Schematic diagram of the potential sites in the PI3 kinase pathway where resistance to single-agent TKIs directed against the EGFR might occur. 1) The phosphatase activity of the PTEN gene product is lost in the majority of GBM, favoring accumulation of the triphosphate form of phosphatylinositol, PIP3; 2) Activating mutations in the PIK3CA gene, controlling the catalytic subunit of PI3K are well-documented, also favoring accumulation of PIP3;3) In addition, increased expression of the phospho-AKT enhancer, PIKE-A, is found. These alterations may occur in isolation or in any combination in GBM.
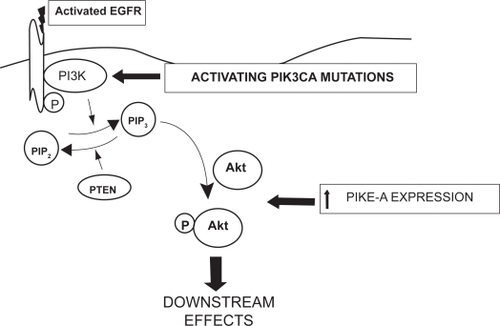
Figure 4 A schematic showing the convergence of the PI3K pathway (through mTOR) and the Ras/MAPK pathway (through ER K) on p70s6K and its effect on translation. Note the inhibitory effect of TSC1 and 2, an area not fully explored in gliomagenesis.
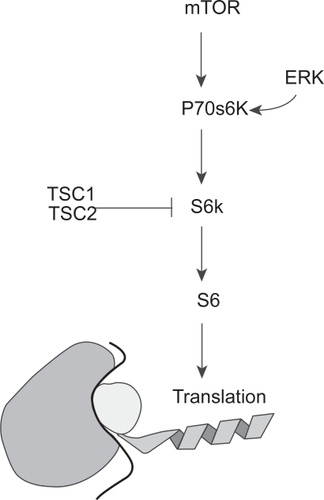
Figure 5 MRI scans showing a typical response of a recurrent GBM to bevacizumab. The image on the right shows the tumor-associated enhancement (large arrow) and accompanying compression of the ventricle by mass effect (small arrow). The image on the right shows the same region following a one month course of bevacizumab. Note the marked reduction in contrast enhancement and mass effect.
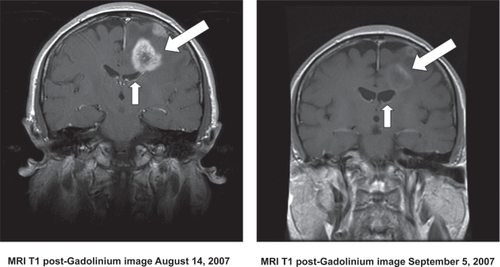
Table 3 Clinical trials of bevacizumab in gliomas
Table 4 A selection of ongoing clinical trials in malignant gliomas