Figures & data
Figure 1 FAK/Src complex mediated signaling pathway.
Abbreviations: ERK, extracellular signal regulated kinase; FA, focal adhesions; MEK, mitogen-activated protein; FAK, focal adhesion kinase; VEGF, vascular endothelial growth factor; MMPs, matrix metalloproteinases.
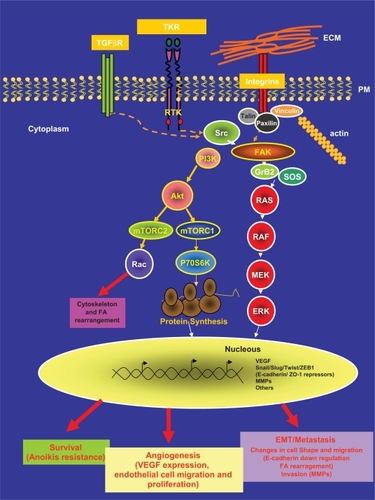
Figure 2 FAK and Src structure.
Notes: A) FAK structure. FAK harbors a central region with kinase activity that is flanked by a N-terminal region that contains the FERM domain and by a C-terminal region that contains the FAT domain. The autophosphorylation in its Y397 residue increases its catalytic kinase activity and allows the binding of specific intracellular proteins. FAK is phosphorylated by Src in its Y576 and Y577 residues allowing its full catalytic activity. In absence of stimulus, the FERM domain works as a negative regulator of FAK activity through its interaction with the kinase domain preventing the phosphorylation in Y397. In response of stimuli, the FERM domain interacts with the cytoplasmic tail of the integrins. Allowing the autophosphorylation of FAK in this tyrosine. The FAT domain, placed in the C-terminal region, mediates the co-localization of FAK with the FA areas through the interaction of FAK with the FA associated proteins talin and paxillin. Two proline-rich domains (PR) mediate the interaction of FAK with SH3 containing proteins such as p130 CAS. B) Src structure. Src is comprised of six domains: a SH4 involved in targeting Src to the plasmatic membrane, a region U that is specific of each Src family member, a SH3 and a SH2 domain involved in the interaction of Src with other intracellular proteins and a SH1 domain involved in ATP and substrate binding. The phosphorylation in Y419 residue of the SH1 domain is required for maximum kinase activity. Placed immediately adjacent to the SH1 domain there is a negative regulatory domain. After phosphorylation of the Y530 residue, in the negative regulatory domain, Src becomes inactive.
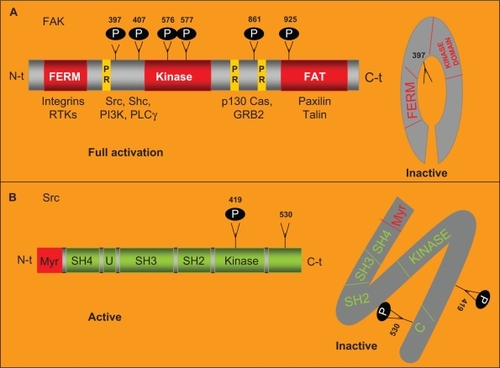
Figure 3 Role of the FAK-Src complex in the malignant progression of solid tumors.
Abbreviations: ECM, extracellular matrix; EMT, epithelial mesenchynal transitions; FAK, focal adhesion kinase; Src, steroid receptor coactivator.
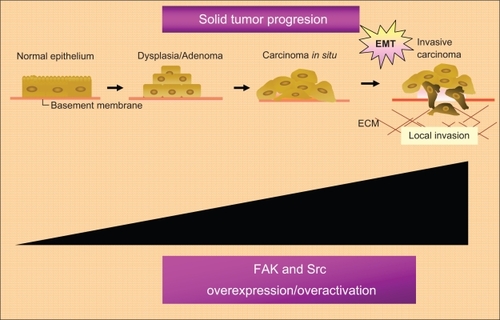
Table 1 Summary of FAK and Src inhibitors under clinical and preclinical development