Figures & data
Figure 1 The cells of the subventricular zone, labeled with the astrocyte marker GFAP (shown in green), line the lateral walls of the lateral ventricles. This is the largest known region of adult neural stem cells in the human brain; it is composed of the deep subcortical white matter A), a periventricular ribbon of astrocytes that can function as neural stem cells B), a dense layer of astrocytic processes C), and the ependymal lining D). Throughout adult life, astrocytes from the subventricular zone exhibit a unique capacity for multipotency and self-renewal in vitro. Copyright © 2005. Reproduced with permission from CitationSanai N, Alvarez-Buylla A, Berger M. 2005. Neural stem cells and the origin of gliomas. N Engl J Med, 353:811–22.
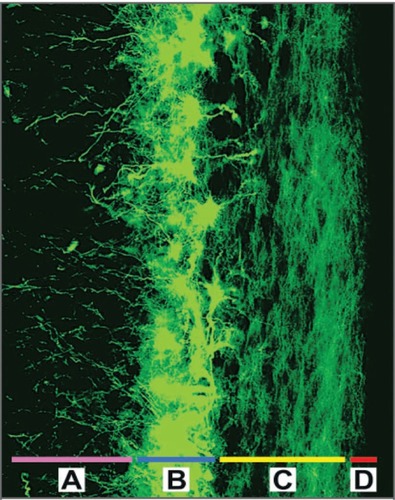
Table 1 Characteristics intrinsic to neural stem cells and gliomas. Copyright © 2005. Reproduced with permission from CitationSanai N, Alvarez-Buylla A, Berger M. 2005. Neural stem cells and the origin of gliomas. N Engl J Med, 353:811–22
Figure 2 Hierarchical organization of the functional compartments in renewing tissues A) The stem-cell compartment (purple), early transiently dividing progenitor compartment (orange), late transiently dividing progenitor compartment (red) and differentiated-cell compartment (green) are schematically described. Cells in the stem-cell and transiently dividing progenitor compartments could be the target of the onco-transformation that leads to the formation of tumour stem cells B) The neural precursors that make up similar functional compartments in the neurogenetic regions of the adult brain and that might be the source of brain tumour stem cells C) The structure of the subventricular zone, showing how these precursors fit and are organized in the germinal neuroepithelium of the largest neurogenetic region of the adult brain.
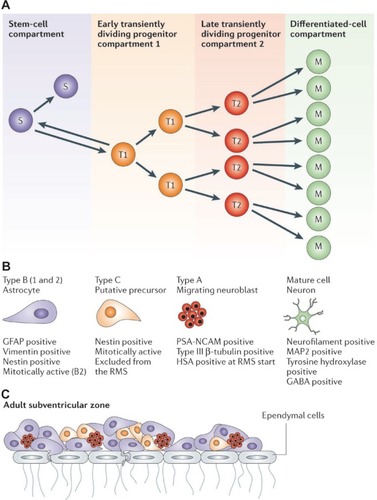
Figure 3 Overview of tumor stem cells in cancer. Cancer stem cells (tumor-initiating cells) divide asymmetrically, resulting in self-renewal of the tumor-initiating cell and production of a daughter cell known as a transient-amplifying cell (progenitor cell). The transient-amplifying cell is not thought to possess self-renewing capabilities, but instead divides indefinitely to contribute to cancer progression. Copyright © 2007. Reproduced with permission from CitationLee J, Herlyn M. 2007. Old disease, new culprit: tumor stem cells in cancer. J Cell Physiol, 213:603–9.
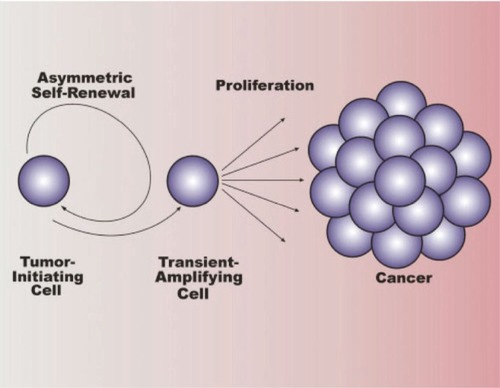
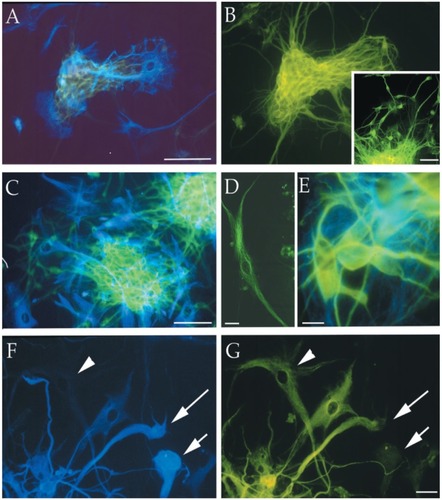
Figure 4 Immunofluorescence photomicrographs of representative MC-PL clones derived from different cortical glial tumor specimens (T1–T3, T6, and T7) processed for double (A, B, C, E, F) or single (B inset, D) labeling for different neural markers. The cells positive for the neuronal marker β-III tubulin are stained green (FITC), and the cells that expressed the astroglial marker GFAP are stained blue (AMCA). The arrowhead in F points out a cell that is not labeled for GFAP, but is immunopositive for β-III tubulin (arrowhead in G). The short arrow in F points out a cell that is immunolabeled for GFAP, but immunonegative for β-III tubulin (short arrow in G). The long arrow in F and G points out a cell that is labeled with both immunomarkers. Scale bars are 10 μm (F and G) and 100 μm (A–E). Copyright © 2002. CitationIgnatova T, Kukekov V, Laywell E, et al. 2002. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia, 39:193–206.