Figures & data
Table 1 Characteristics of currently available drugs for malaria prevention
Table 2 Characteristics of future prophylaxis drugs
Figure 1 Clinical development of new drugs for malaria prevention.
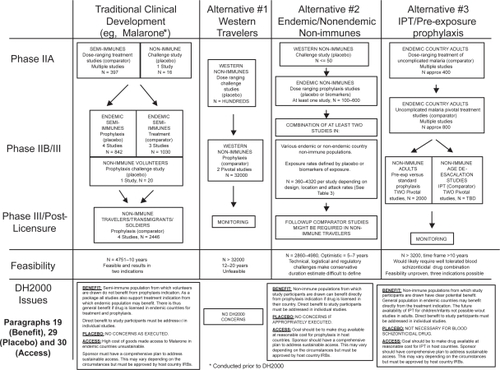
Table 3 Possible phase III-equivalent study designs