Figures & data
Figure 1 Proposed model for opioid receptor-mediated analgesia at peripheral terminals of primary sensory neurons. Activation of opioid receptors by endogenous or exogenous opioid agonists promotes G-protein coupling. Opioid receptor-coupled G-proteins directly activate inwardly rectifying K+ channels (GIRK+), inhibit voltage-dependent Ca2+ channels, and inhibit adenylyl cyclase (AC). Opioid receptors indirectly activate phospholipase C (PLC), the mitogen-activated protein kinase (MAPK) cascade, and large conductance Ca2+-activated K+ channels by utilizing other intermediary messenger systems.
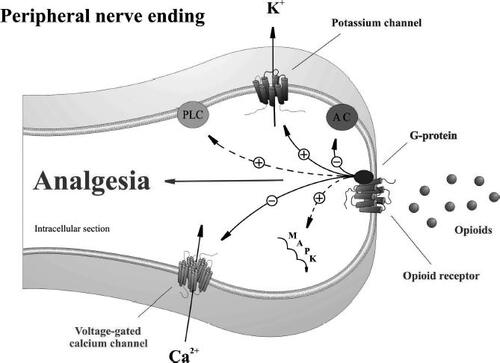
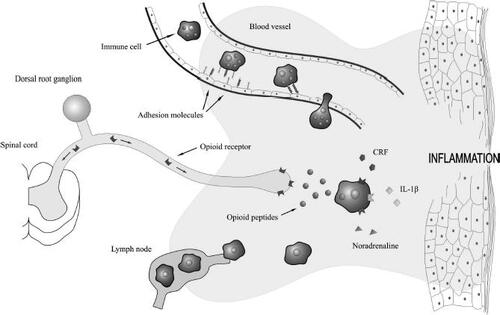
Figure 2 Endogenous opioid peptides are released by immune cells to reduce inflammatory pain. Adhesion molecules expressed on immune cells and inflamed endothelium coordinate the migration of circulation immune cells into inflamed tissue. The proinflammatory mediators corticotropin-releasing factor (CRF) and interleukin-1β (IL-1β), as well as the sympathetic neurotransmitter, noradrenaline, stimulate immune cells to secrete their opioid peptides. These peptides activate opioid receptors located on the peripheral ends of sensory neurons and effectively reduce inflammatory pain. Immune cells, devoid of their opioid contents, then continue their passage to neighbouring lymph nodes.