Figures & data
Table 1 Morbidity associated with untreated chronic pain (CitationAPS 1996)
Figure 1 ACR treatment guidelines for pharmacological management of noncancer chronic pain systemic agents (CitationACR 2000).
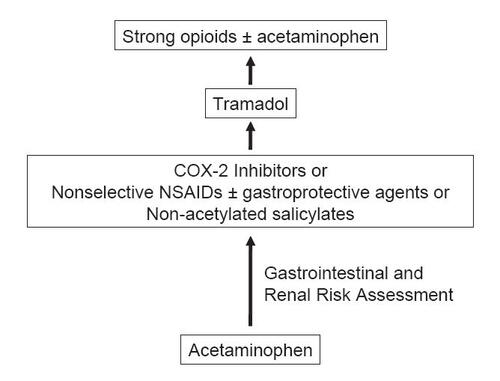
Table 2 Limitations of pharmacologic therapy for chronic pain
Figure 2 Structure of tramadol extended-release: (±) cis-2-[(dimethylamino) methyl]-1-(3-methoxyphenyl) cyclohexanol hydrochloride (CitationUltram PI 2004).
![Figure 2 Structure of tramadol extended-release: (±) cis-2-[(dimethylamino) methyl]-1-(3-methoxyphenyl) cyclohexanol hydrochloride (CitationUltram PI 2004).](/cms/asset/88bcd6b9-11ab-4948-8317-f843c15c114e/dtcr_a_12160413_f0002_b.jpg)
Figure 3 Pharmacokinetics of tramadol ER 200 mg once daily (qd) versus tramadol IR 50 mg every 6 hours (q6h) (mean steady-state tramadol plasma concentrations in healthy subjects on day 8 post dose) (CitationUltram PI 2006).
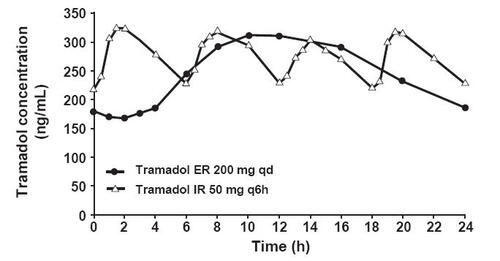
Figure 4 WOMAC pain responder analysis: patients with moderate to moderately severe pain due to osteoarthritis of the knee and/or hip achieving various levels of response with tramadol ER. Patients in the 100 mg and 200 mg treatment groups demonstrated a statistically significant improvement in pain compared with placebo (CitationUltram PI 2006).
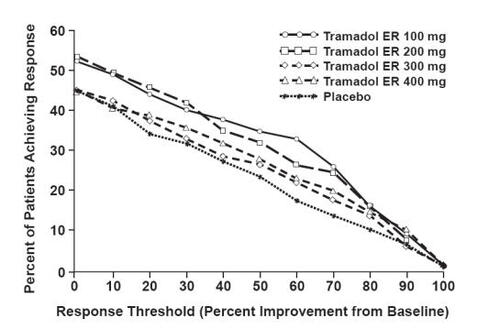
Figure 5 Efficacy of tramadol ER in patients with osteoarthritis: mean change from baseline in Arthritis Pain Intensity VAS assessed through 12 weeks. Reprinted from CitationBabul N, Noveck R, Chipman H, et al. 2004. Efficacy and safety of extended-release, once-daily tramadol in chronic pain: a randomized 12-week clinical trial in osteoarthritis of the knee. J Pain Symptom Management, 28:59–71. Copyright © 2004 with permission from Elsevier.
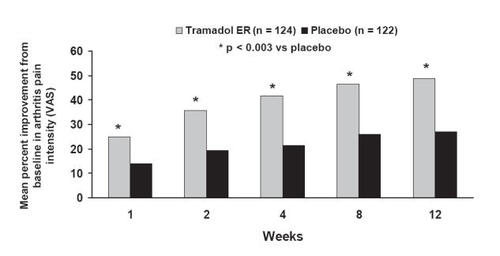
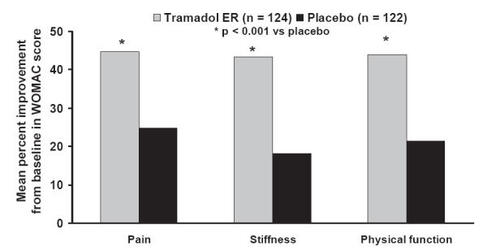
Figure 6 Efficacy of tramadol ER in patients with osteoarthritis using the WOMAC pain, stiffness, and physical function subscales. Mean percentage change in subscales were assessed from baseline to 12 weeks of treatment. Tramadol ER significantly improved pain, stiffness, and physical function subscales, compared with placebo (p < 0.001). Reprinted from CitationBabul N, Noveck R, Chipman H, et al. 2004. Efficacy and safety of extended-release, once-daily tramadol in chronic pain: a randomized 12-week clinical trial in osteoarthritis of the knee. J Pain Symptom Management, 28:59–71. Copyright © 2004 with permission from Elsevier.