Figures & data
Table 1 International prognostic scoring system (IPSS) score and prognosis
Figure 2 Time to acute myeloid leukemia (AML) or death: (A) all patients; (B) treatment-naïve patients; (C) International Prognostic Scoring System subgroups intermediate-2 to high-risk patients. (Reprinted, with permission, from CitationKantarjian et al 2006).
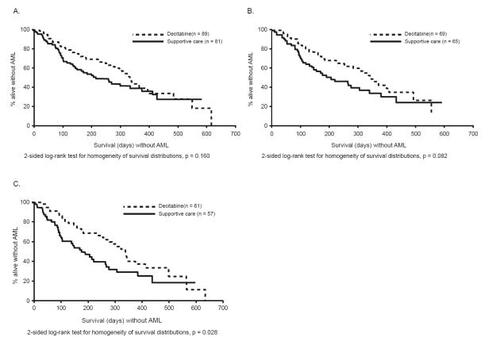
Table 2 Response to decitabine (ITT) using the FDA approved dose of 15 mg/m2 over 3 hours every 8 hours ×3 days every 6 weeks (Adapted, with permission, from CitationKantarjian et al (2006))
Figure 3 Percentage of patients red blood cell (RBC) transfusion free per cycle (Reprinted, with permission, from CitationKantarjian et al 2006).
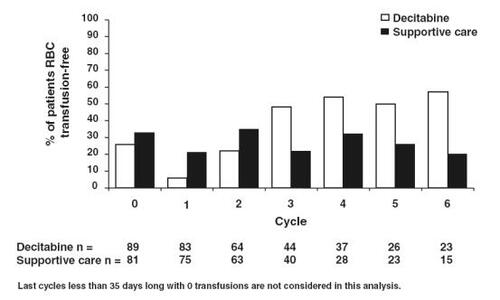
Table 3 Most common adverse events of decitabine
Table 4 Efficacy and side effects of three alternative decitabine dosing schedules (Adapted, with permission, from CitationKantarjian et al(2007))
Table 5 Decitabine dosing schedules