Figures & data
Figure 1 Percentage of patients achieving 75% improvement in Psoriasis Area and Severity Index response at week 12 in the phase II and phase III trials. Because the phase II trial did not have week 16 data (the primary endpoint for the phase III trials), we compared the week 12 PASI 75 for all three clinical trials.
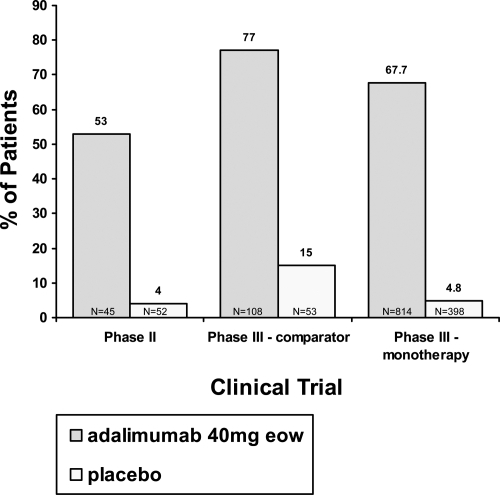
Figure 2 Clinical and histological response of subject in phase II study of adalimumab after 12 weeks. Clinical photos target lesion at day 0 (A) and day 84 (B) and representative histological specimens at day 0 (C) and day 84 (D).
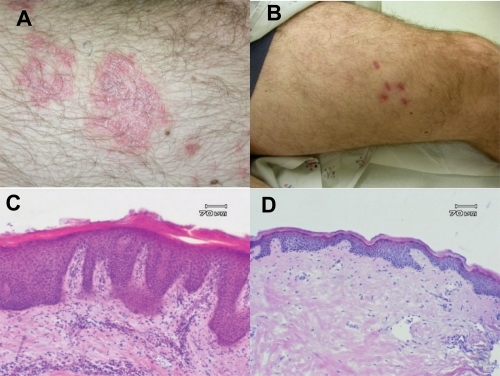
Table 1 Adverse events in adalimumab psoriasis trials