Figures & data
Table 1 Clinical trials evaluating the efficacy of solifenacin in the treatment of overactive bladder
Figure 2 Reduction from baseline in the number of micturition and urgency episodes in 24 hours, and episodes of nocturia in patients receiving placebo, 5 mg, or 10 mg of solifenacin in a phase 3 trial (drawn from data of CitationCardozo et al 2004).
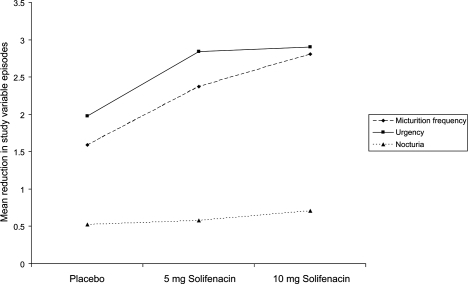
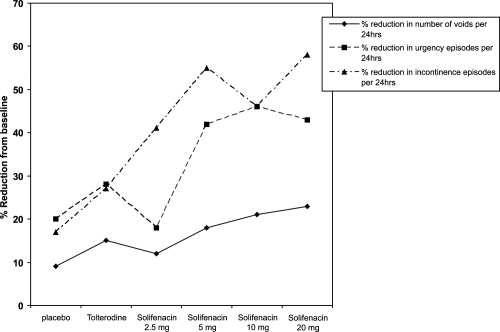
Figure 3 The percentage change from baseline in the mean number of urgency, incontinence, and urge incontinence episodes and mean voids in a 24-hour period (Drawn from data of CitationChapple et al 2004b).
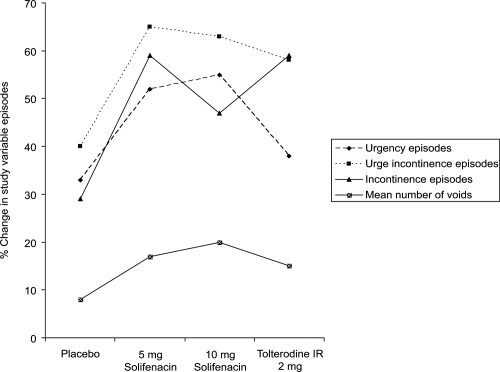
Figure 5 Median percentage reductions in frequency, urgency, and nocturia in long-term solifenacin treated patients. Reprinted with permission from Haab F, Cardozo L, CitationChapple C, et al. 2005. Long term open-label solifenacin treatment associated with persistence with therapy in patients with overactive bladder syndrome. Eur Urol, 47:376–84. Copyright © 2005 Elsevier.
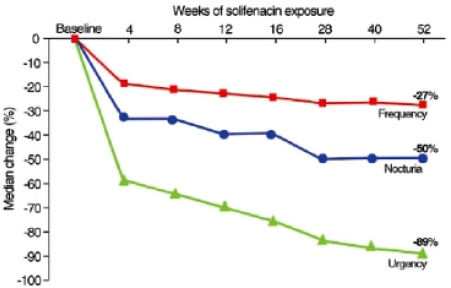
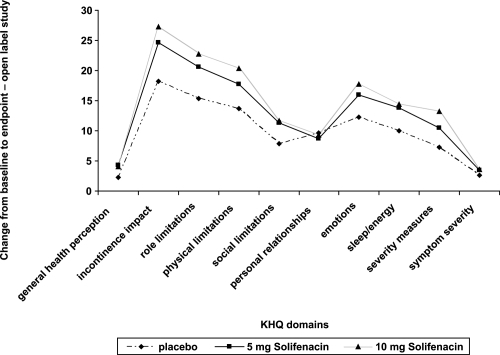
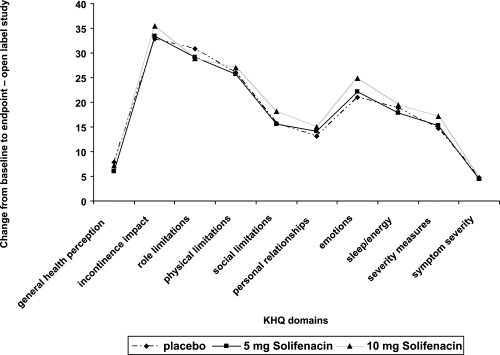
Figure 6 Pooled changes from baseline in the KHQ domains for 2 of the phase 3 trials for solifenacin.