Figures & data
Figure 1 In multi-step studies, increased minimum inhibitory concentrations (MICs) to retapamulin were seen among Staphylococcus aureus isolates, but required more passages compared with mupirocin or fusidic acid; figure shows a summary of the 12 S. aureus isolates tested. Drawn from data of Kosowska-Shick et al.Citation19
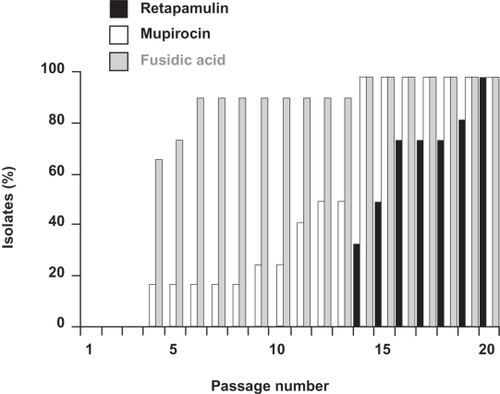
Figure 2 Clinical success rates at follow-up in the per-protocol clinical populations from randomized controlled clinical trials comparing topical retapamulin, 1%, twice daily for 5 days, with placebo in the treatment of impetigo and oral cephalexin, 500 mg, twice daily for 10 days, in the treatment of secondarily infected traumatic lesions (SITLs) and secondarily infected dermatitis (SID). Drawn from data of Koning et al,Citation11 Free et al,Citation30 and Parish et al.Citation31
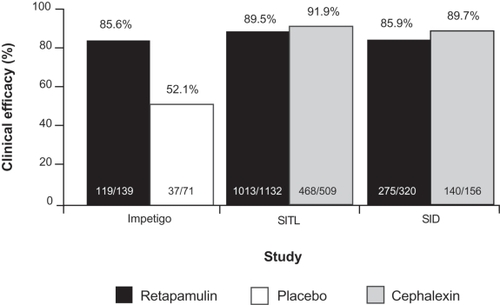
Figure 3 Compliance with retapamulin was greater than that for cephalexin in patients with secondarily infected traumatic lesions treated with topical retapamulin, 1%, twice daily for 5 days, or with oral cephalexin, 500 mg, twice daily for 10 days; figure shows pooled data from two identical randomized controlled clinical trials (Study 030A and 030B) for intent-to-treat patients who were 80%–120% compliant with study treatment.Citation30 For secondarily infected dermatitis (Study 032), there was no difference in compliance between the two treatment arms.Citation31 Patients ≥13 years of age received cephalexin capsules; those who were younger received cephalexin suspension. Drawn from data of GlaxoSmithKline.Citation36
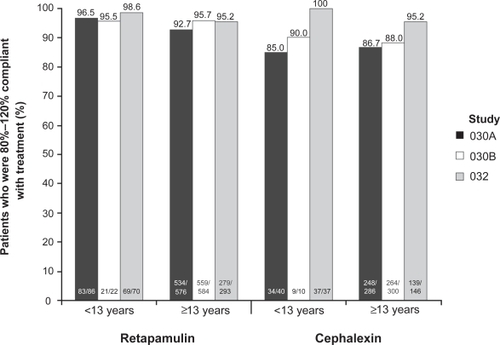
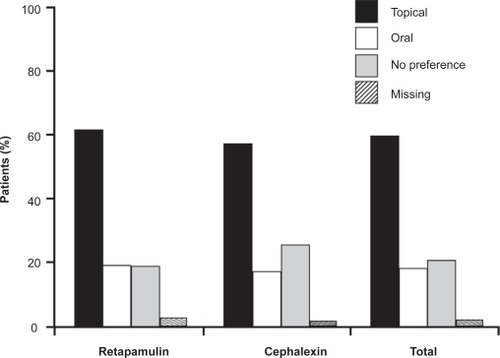
Figure 4 Patients with secondarily infected dermatitis treated with topical retapamulin, 1%, twice daily for 5 days, or with oral cephalexin, 500 mg, twice daily for 10 days, recorded a marked preference for topical therapy over oral therapy in both treatment arms. Drawn from data of Parish et al.Citation31