Figures & data
Table 1 Trial design and key efficacy results from the ESCALATOR and EPIC studies
Figure 1 Mean liver iron concentration (LIC) and median serum ferritin at 1 year and end of study. Reproduced with permission. Taher A, El-Beshlawy A, Elalfy M, et al. Haematologica. 2009;94(Suppl 2):abstr 209.Citation25 Obtained from Haematologica/the Hematology Journal website http://www.haematologica.org with kind permission of the Ferrata Storti foudation, Pavia, Italy.
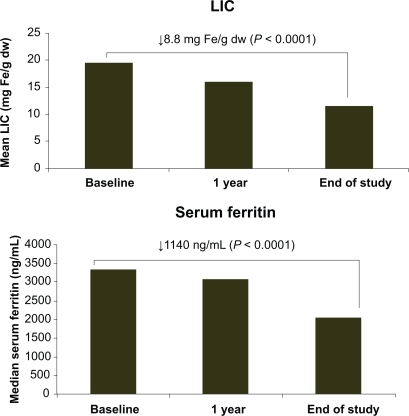
Figure 2 Mean dose and median serum ferritin during long-term deferasirox treatment in patients with β-thalassemia.Citation28
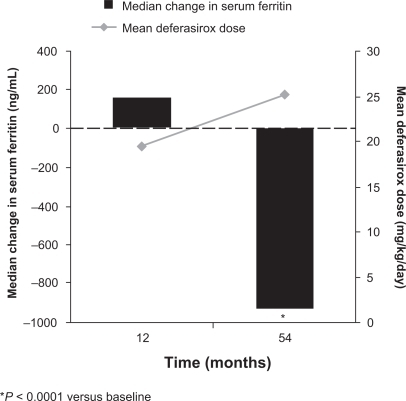
Figure 3 Median change from baseline in serum ferritin in patients enrolled in the EPIC study.Citation26
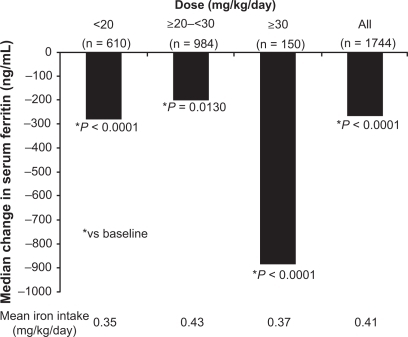
Table 2 Efficacy of deferasirox across various transfusion-dependent anemias: data from the EPIC trial
Figure 4 The effect of deferasirox on the mean cardiac T2* by baseline T2* in patients A) with cardiac iron overload; B) with normal cardiac levels.Citation41,Citation42
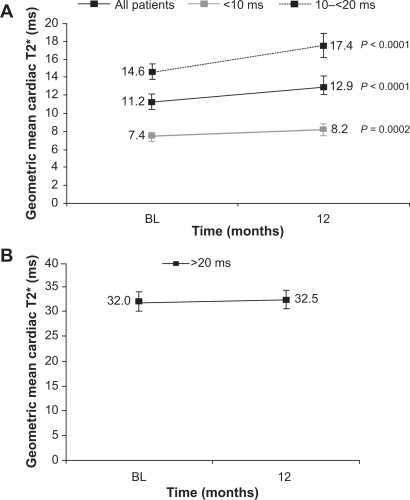
Figure 5 Mean labile plasma iron (LPI) taken pre-administration and 2 hours post-administration of deferasirox reproduced with permission. Daar S, Pathare A, Nick H, et al. Reduction in labile plasma iron during treatment with deferasirox, a once-daily oral iron chelator, in heavily iron-overloaded patients with β-thalassaemia. Eur J Haematol. 2009;82(5):454 457.Citation64 © Wiley-Blackwell 2009.
*Versus pre-administration at baseline.
LPI data are from 13 patients except †n = 11 ‡n = 12 due to lost samples.
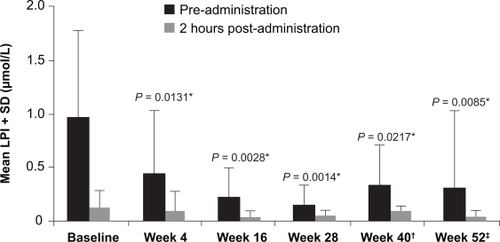
Table 3 Most common drug-related adverse events during a median 4.5-year treatment period with deferasirox in 472 patients with β-thalassemiaCitation28Table Footnote*
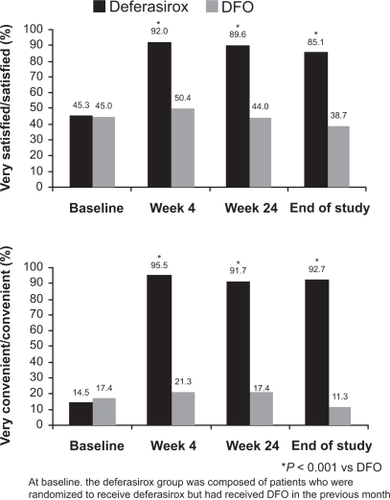
Figure 6 Overall treatment satisfaction with iron chelation therapy in patients previously treated with deferoxamine (DFO). Reprinted from Clinical Therapeutics. Cappellini MD, Bejaoui M, Agaoglu L, et al. Prospective evaluation of patient-reported outcomes during treatment with deferasirox or deferoxamine for iron overload in patients with β-thalassemia. Clin Ther. 2007;29(5):909 917Citation78 with permission from Excerpta Medica, Inc.