Figures & data
Figure 1 Adjusted coronary heart disease mortality rates according to HDL-cholesterol and LDL-cholesterol levels during 21 years of follow-up in 8586 Israeli men without coronary heart disease at baseline. Cut-off values to define low/high lipid levels were 0.9 mmol/L for HDL-cholesterol and 5.2 mmol/L for total-cholesterol. RR: risk ratios compared with corresponding low HDL-cholesterol group (adjusted for age, systolic blood pressure, smoking, and diabetes). Drawn from data presented by CitationGoldbourt et al (1997).
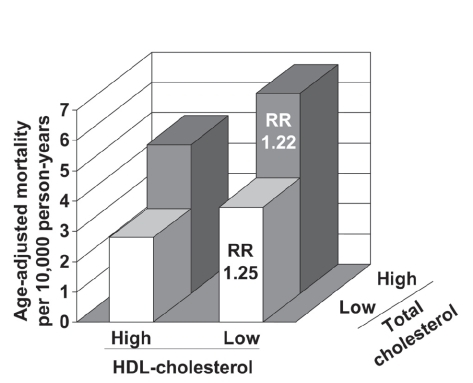
Table 1 Prevalence of low HDL-cholesterol in the Pan-European Survey of HDL-cholesterol (CitationBruckert et al 2005a)
Figure 2 Dose-related effect of Niaspan® (500–3000 mg/day) on lipid parameters in a 25-week, double-blind, randomized trial in 131 patients with hyperlipidemia. *p < 0.05 vs placebo. Effects of placebo have been omitted for clarity. Drawn from data presented by CitationGoldberg et al (2000).
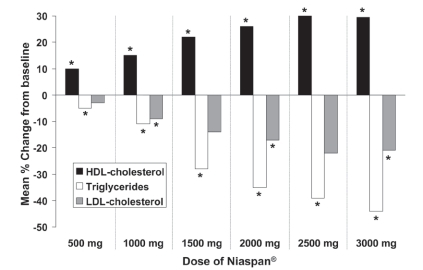
Table 2 Effects of Niaspan® on lipoprotein (a) (Lp(a)) in controlled clinical trials
Figure 3 Comparison of effects on lipids of combinations of nicotinic acid with a statin in comparison with a combination of a statin with ezetimibe or rosuvastatin monotherapy in a 12-week, open-label, randomized trial in 292 patients indicated for LDL-cholesterol lowering therapy. Patients received rosuvastatin (20–40 mg), rosuvastatin plus Niaspan® (10/1000 mg or 20/1000 mg), atorvastatin plus Niaspan® (20/1000 mg or 40/2000 mg), or simvastatin plus ezetimibe (20/10 mg or 40/10 mg). Significance values are from ANOVA across groups. Drawn from data presented by McKenney et al (2006).
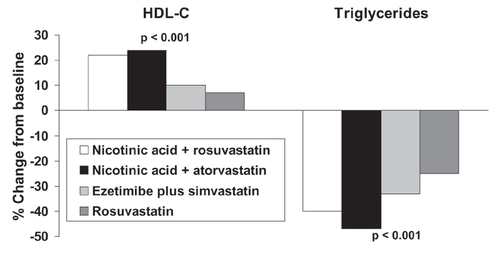
Figure 4 Long-term (96 weeks) effects of Niaspan® (up to 3000 mg/day) on the lipid profile in patients with dyslipidemia, with (n = 122) or without (n = 225) concomitant statin administration. Drawn from data presented by CitationGuyton and Capuzzi (1998).
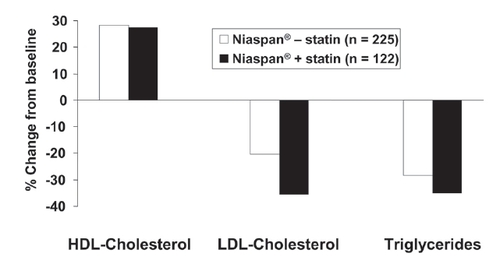
Figure 5 Flushing with Niaspan® in the NAUTILUS trial, a 15-week evaluation of Niaspan® at doses up to 2000 mg/day in 566 patients with dyslipidemia and low HDL-cholesterol managed in the usual care setting. Reproduced with permission from CitationVogt A, Kassner U, Hostalek U, et al 2006. Evaluation of the safety and tolerability of prolonged-release nicotinic acid in a usual care setting: the NAUTILUS study. Curr Med Res Opin, 22:417-25.
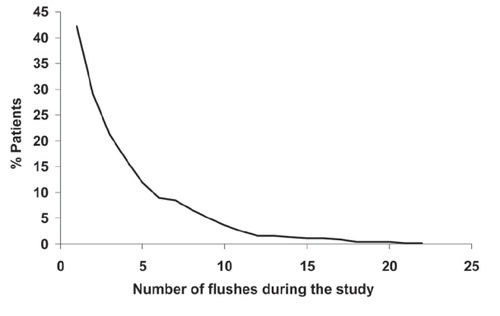
Figure 6 Mean changes in HDL-cholesterol and triglycerides in subgroups of the NAUTILUS population based on gender, presence/absence of the metabolic syndrome (MS), hypercholesterolemia (HC), isolated low HDL-cholesterol (ILH), receipt/non-receipt of statins, presence/absence of diabetes mellitus (DM), and age. Drawn from data presented by CitationVogt et al 2006a, Citationb, Citationc, Citationd.
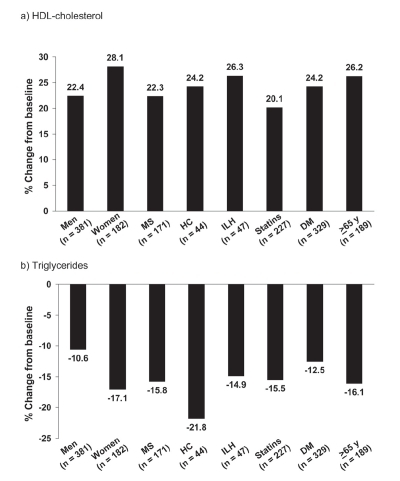
Figure 7 Treatment assignments in the double-blind, randomized, ARBITER 2 study and its open-label follow-up study, ARBITER 3. Numbers of patients shown are those completing each phase. The duration each phase trial was 1 year; the Niaspan® daily dose was 1000 mg. Adapted with permission from CitationTaylor AJ, Lee HJ, Sullenberger LE. 2006. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin, 22:2243-50.
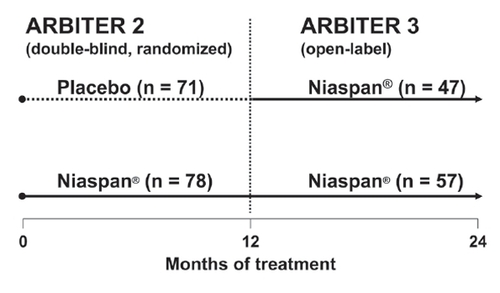
Table 3 Effects of Niaspan® on atherosclerosis in the ARBITER 2 study and in a pooled analysis of data from the ARBITER 2 and ARBITER 3 studies