Figures & data
Table 1 Pharmacokinetic properties of trandolapril (T) in healthy volunteers
Table 2 Pharmacokinetic interaction between oral trandolapril or verapamil SR and other agents when administered concomitantly (only clinically relevant interactions are cited)
Figure 1 Among the patients who did not have diabetes at baseline (8098 in the verapamil SR group and 8078 in the atenolol group) addition of trandolapril in verapamil SR strategy was associated with more protection from the development of new onset diabetes than that in the atenolol/hydrochlorothiazide strategy arm. When trandolapril 2 mg was added to verapamil 180 mg and trandolapril 4 mg to verapamil 240 mg the hazard ratio for development of new onset diabetes was 0.56 (0.98–1.64, confidence interval [CI] 95%), and 0.58 (0.44–0.78, CI 95%) respectively.
![Figure 1 Among the patients who did not have diabetes at baseline (8098 in the verapamil SR group and 8078 in the atenolol group) addition of trandolapril in verapamil SR strategy was associated with more protection from the development of new onset diabetes than that in the atenolol/hydrochlorothiazide strategy arm. When trandolapril 2 mg was added to verapamil 180 mg and trandolapril 4 mg to verapamil 240 mg the hazard ratio for development of new onset diabetes was 0.56 (0.98–1.64, confidence interval [CI] 95%), and 0.58 (0.44–0.78, CI 95%) respectively.](/cms/asset/1b2975fe-1886-4ed7-b9a9-148f91cc01aa/dvhr_a_12187373_f0001_c.jpg)
Figure 2 The BErgamo NEphrologic DIabetes Complications Trial (BENEDICT) is a multicenter double-blind, randomized study designed to assess whether the angiotensin converting enzyme inhibitor trandolapril and the non-dihydropyridine calcium-channel blocker verapamil, alone or in combination, prevent microalbuminuria in subjects with hypertension, type 2 diabetes mellitus, and normal urinary albumin excretion. The Kaplan-Meier curves show the percentages of subjects with microalbuminuria during treatment with trandolapril/verapamil or placebo. The difference between the two groups adjusted for pre-specified baseline covariates was significant (p = 0.01) according to the accelerated failure (A.F.)-time model.
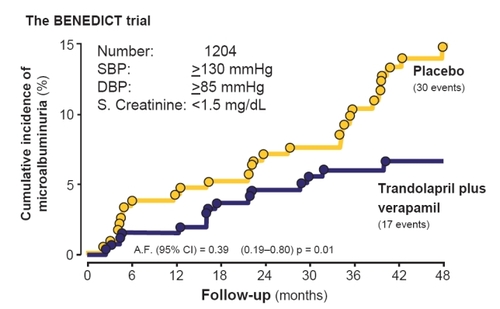
Figure 3 Patients who developed microalbuminuria throughout the study period of the BENEDICT trial according to follow-up systolic blood pressure (SBP). These are patients with type 2 diabetes, arterial hypertension, and normoalbuminuria at baseline. Effective SBP reduction below the median (<139.16 mmHg) has specific and independent protective effects against the development of microalbuminuria. The risk reduction for microalbuminuria that was achieved by angiotensin converting enzyme inhibitor (ACEi) therapy in patients with follow-up SBP above the median (≥139.16 mmHg) was highly significant even after adjustment for baseline covariates and concomitant treatment with non-dihydropyridine calcium channel blockers. Thus ACEi therapy had a further protective effect, in particular when SBP was less effectively controlled (inset).
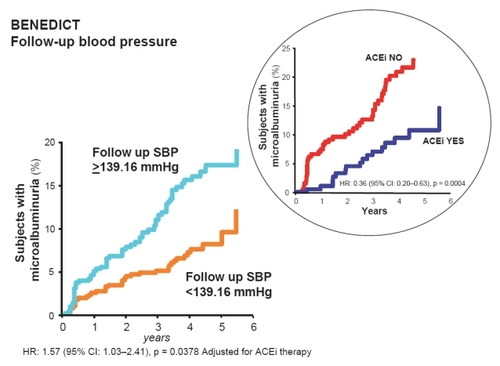
Figure 4 In the BENEDICT study, the extent of systolic blood pressure (SBP) reduction had a specific and independent effect against the development of microalbuminuria. Angiotensin converting enzyme inhibitor (ACEi) therapy had a further protective effect, in particular when the SBP was less effectively controlled (inset).
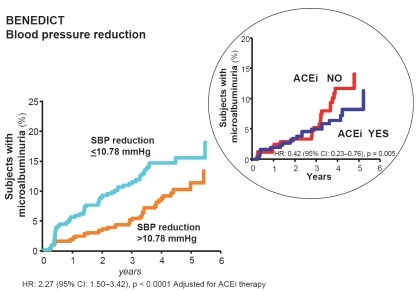
Table 3 Number (%) of patients on different antihypertensive treatments on trial according to systolic blood pressure (SBP), diastolic BP (DBP), mean arterial pressure (MAP), and pulse pressure reduction above or below the median