Figures & data
Table 1 Commercial recombinant FVIII therapies
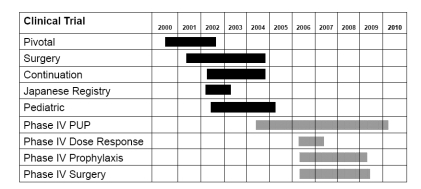
Table 2 Global clinical program of ADVATE®
Figure 1 PK comparisons of Recombinate® and ADVATE®. Post-infusion factor VIII levels (logarithmically adjusted for illustrative purposes) over time were similar with R-FVIII and ADVATE®. Data shown represent the natural logarithm FVIII activity (Ln) as a function of time in hours (h). Reprinted with permission from CitationTarantino MD, Collins PW, Hay CR, et al 2004. Clinical evaluation of an advanced category antihaemophilic factor prepared using a plasma/albumin-free method: pharmacokinetics, efficacy, and safety in previously treated patients with haemophilia A. Haemophilia, 10:428–37. Copyright © 2004 Blackwell Publishing.
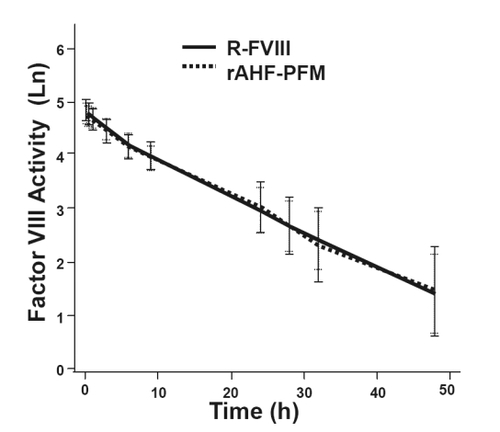
Figure 2 Mean annual total bleed episode rate per regimen. Subjects on prophylactic regimens experienced fewer bleeding episodes than those using on-demand treatment.
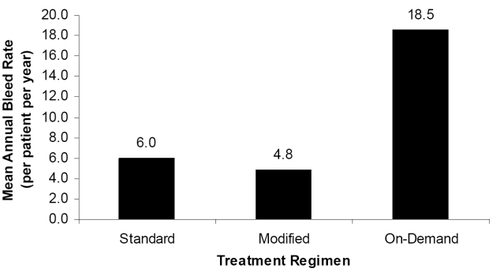
Table 3 Predicted and actual blood loss associated with surgical procedures
Figure 3 Global clinical program study plan. Five Phase II/III clinical studies have been completed (dark bars), while four additional Phase IV studies are in progress as of 2007 (hatched bars).