Figures & data
Figure 1 Schematic tissue factor-independent, platelet-dependent model of primary hemostatic plug formation in Glanzmann’s thrombasthenia (GT) platelets deficient in membrane glycoprotein (GP) IIb-IIIa (integrin aIIbβ3). FVIIa-tissue factor (TF) complex on TF-bearing cells at the site of vascular injury activates FX to FXa, which in turn complexes with FVa on the TF-bearing cells to initiate generation of a small amount of thrombin (FIIa) from prothrombin (FII). This initially generated thrombin is not sufficient to allow fibrin formation, but is sufficient to activate the GT platelets, causing degranulation and release of FV. FVIIa binds to activated platelets weakly. At high concentration (eg, high dose rFVIIa therapy), the bound FVIIa can directly activate FX to FXa to mediate generation of high concentration of thrombin (thrombin burst). The augmented thrombin generation results in increased number of activated platelets deposited (adhesion) to the wound site, and increased available platelet procoagulant surface to facilitate more thrombin generation and more platelet activation. The augmented thrombin generated also converts fibrinogen to fibrin. Activated GT platelets cannot utilize fibrinogen for aggregation reaction as they lack the fibrinogen receptor integrin aIIbβ3. However, binding of fibrin/polymeric fibrin to an as yet unidentified platelet surface receptor can mediate aggregation of the GT platelets at the wound site (even though less potent than fibrinogen mediated aggregation of normal platelets) resulting in primary hemostatic plug formation. Adapted from CitationPoon (2006).
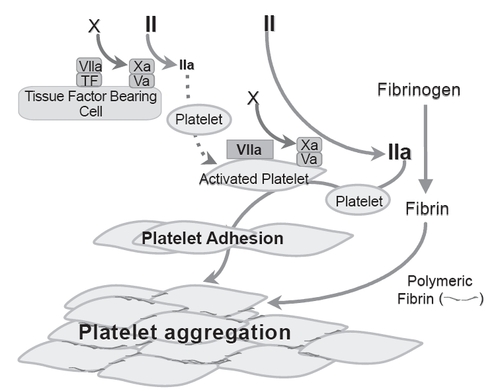
Table 1 Outcome of rFVIIa treatment for 34 surgical procedures and 108 bleeding episodes in patients with Glanzmann’s thrombasthenia (data from CitationPoon et al (2004))
Table 2 Invasive procedures (N = 29) successfully treated with rFVIIa as first-line therapy (3 non-evaluableTable Footnotea episodes and 2 failuresTable Footnoteb not included) (data from CitationPoon (2004))