Figures & data
Figure 1 Schematic showing the current understanding of important players and pathways contributing to diabetic nephropathy/diabetic kidney disease. Point of action for telmisartan with dual ARB plus selective PPAR-γ agonist properties is highlighted. Positive indicates action favoring DKD and negative indicates protection against DKD.
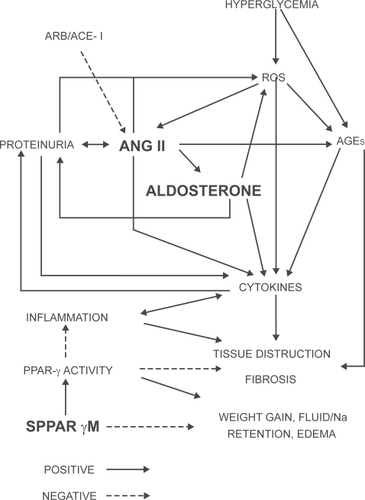
Table 1 Recent clinical trials leading up to the AMADEO study
Table 2 Pharmacokinetics of ARBsCitation37,Citation38,Citation39
Figure 2 Inherent PPAR-γ agonist activity of various ARBs. Importantly, full PPAR-γ agonists, ie, thiazolidinediones typically have agonist activity in the 150 to 200 range. Drawn from data of.Citation36
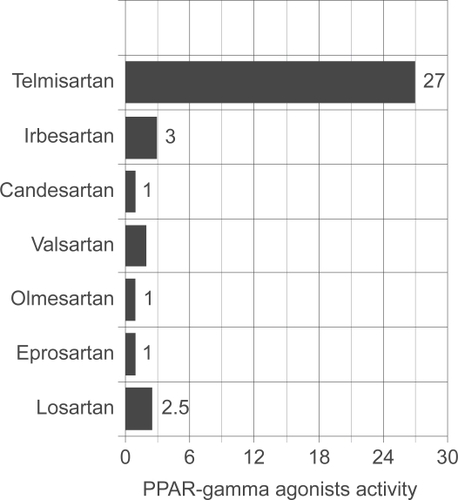
Figure 3 Primary endpoint from AMADEO trial. Patients in the two treatment groups (telmisartan vs losartan) started with the same mean UPC of 2000 mg/g creatinine. Note that the telmisartan arm had significantly lower mean UPC at every time-point over the course of the next year when compared to losartan treated patients. Drawn from data of.Citation2
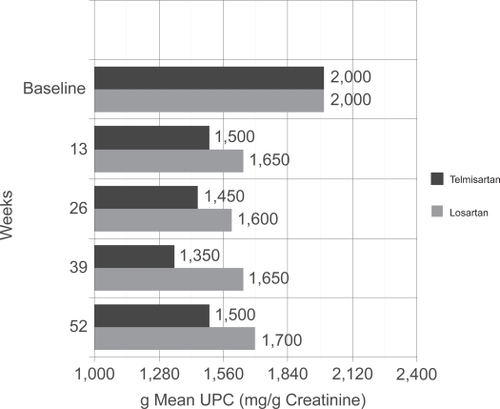
Table 3A Primary endpoint of AMADEO trialCitation2
Table 3B Secondary endpoint of AMADEO trialCitation2