Figures & data
Table 1 Circumferences (micrometers) of the aortas isolated from four experimental groups
Figure 1 High incidence of AAA formation in C57/B6J mice treated with both AngII and BaP. Representative HE-stained tissue sections of suprarenal segments (1 cm above left renal artery) of the aortas from control and treated mice were shown (A). We measured the circumference of aortic rings from four experimental groups using the computer program of NIH Image J. AAA was defined as circumferences that were increased by more than 50% of the average circumference in the control group (946 ± 69 micrometers). Seven mice from the group treated with both AngII/BaP and two from the AngII-treated group showed AAA formation with circumferences greater than 1417 micrometer (B, each diamond represents a different mouse). Differences in the occurrence of AAA formation between the AngII-treated group and the group treated with both AngII and BaP was statistically significant (P < 0.05). Histology of the aortic wall showed severe damage to the medial layer, breakage of elastic lamella, hematoma, and aortic rupture (indicated by the arrow) in the mouse group treated with both AngII and BaP (A). The aortic wall at the location of rupture was significantly thinner, compared to other parts of the aortic wall. A damaged and thinner aortic wall (broken arrows) was also observed in aortic rings harvested from mice treated with AngII and mice treated with both AngII and BaP.
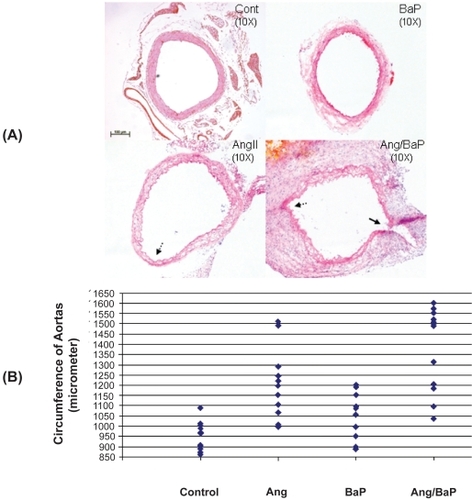
Figure 2 High incidence of AAA rupture and hematoma formation in C57/B6J mice treated with both AngII and BaP. Representative photomicrographs of aortic segments from heart to kidney, harvested from control and treated mice were shown (A). Five mice from the group treated with both AngII and BaP and one in the AngII-treated group showed AAA rupture and hematoma formation (solid arrow). No arterial rupture or hematoma formation was observed in the control group or in the group treated with BaP alone. In mice treated with AngII/BaP, the yellowish stain surrounding tissues (indicated by broken arrow) was tightly attached to the adventitial layer of the aorta (A). The difference in occurrence of AAA rupture and hematoma formation between the AngII-treated group and the group treated with both AngII and BaP was statistically significant (P < 0.05) (B).
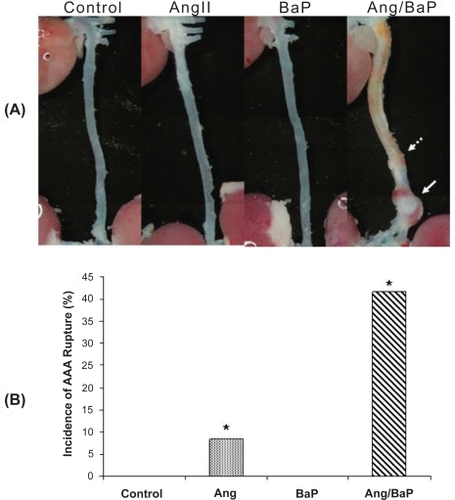
Figure 3 Damage of the arterial wall by AngII or BaP treatments. We show representative images of the aortic wall at the suprarenal segment of the aortas obtained from control, AngII-treated, and BaP-treated mice. Compared to control animals, the medial layer of aortas from AngII-treated mice and BaP-treated mice was shrunken; elastic lamella lost fine lines and instead degraded into loosely defined lines. These changes were particularly severe in aortas from BaP-treated mice, with fewer cells (VSMCs) seen in the medial layer. In adventitia, there were some cells with a rounded-shaped nucleus, resembling inflammatory cells. Although the medial layer of the aortas from BaP-treated mice was severely damaged, the adventitia of the arteries was thicker.
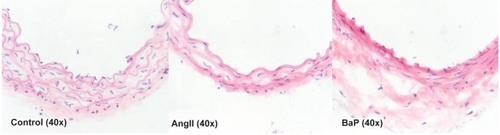
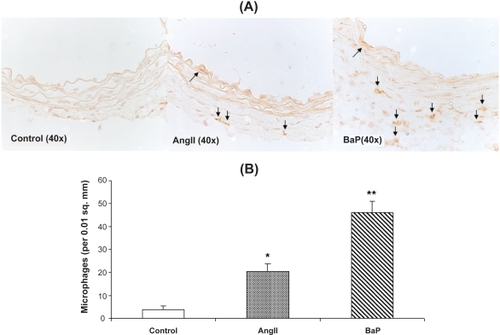
Figure 4 Macrophage infiltration into the aortic wall caused by AngII or BaP treatments. We present the images of immunohistochemical staining with macrophage F4/80 antibody of aortic sections from control, AngII-treated and BaP-treated mice. Macrophage infiltration within the aortic wall (brown staining) was quantified by counting positively stained cells per area of 0.01 square millimeters (n = 6) (B). The results show that both AngII and BaP caused macrophage infiltration into the aortic wall, but the response was more intense in BaP-treated mice.