Figures & data
Figure 2 The triumvirate: insulin resistance in liver and muscle with impaired insulin secretion represent the three core defects in T2DM. Reproduced with permission from DeFronzo RA. Lilly lecture. The triumvirate: Beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1998;37:667–687.Citation16 Copyright © 1998 American Diabetes Association.
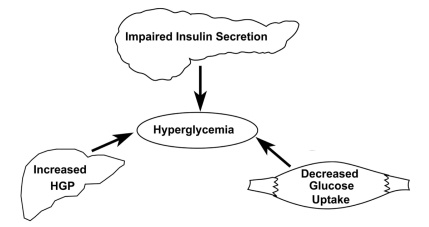
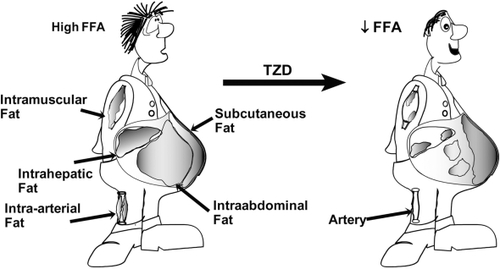
Figure 3 Effect of thiazolidinedione (TZD) treatment on beta cell function.
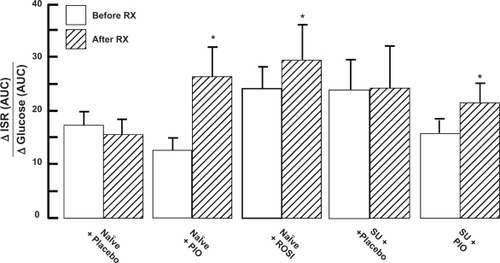
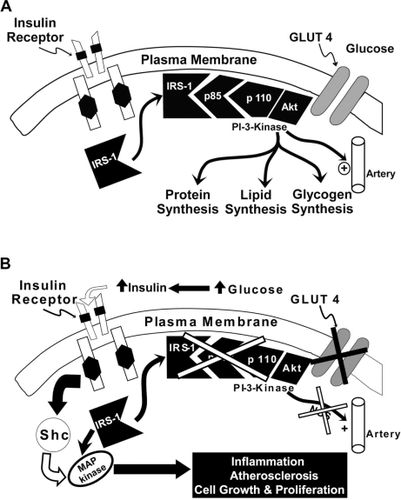
Figure 4 Pioglitazone positively affects the insulin signaling system resulting in improved glycemic control, generation of nitric oxide and decreased MAP kinase pathway activation.
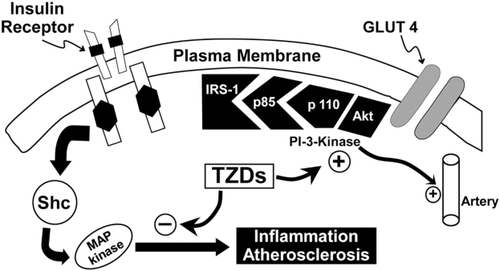
Figure 6 Summary of studies examining the effect of thiazolidinediones (TZDs) versus placebo or versus active-comparator on HbA1C in type 2 diabetes subjects.
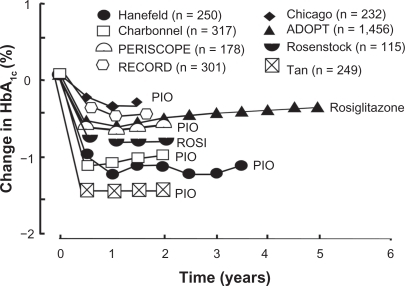
Figure 7 GLP-1 levels decline as glucose tolerance deteriorates A), whereas GIP levels are normal or elevated in patients with type 2 diabetes mellitus B).Citation86–Citation88
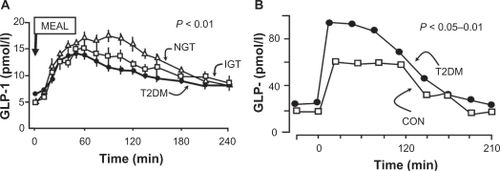
Figure 8 Percentage (%) of subjects achieving select HbA1c targets with alogliptin in Phase 3 trials.Citation135–Citation138
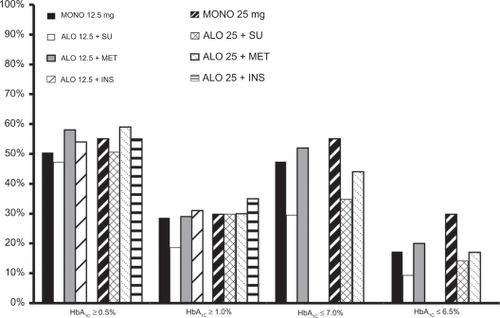
Table 1 Phase III alogliptin trials and alogliptin–pioglitazone combination studies
Figure 9 Necessity for hyperglycemic rescue* in Phase III trials with alogliptin.Citation135–Citation138
*see text for definitions
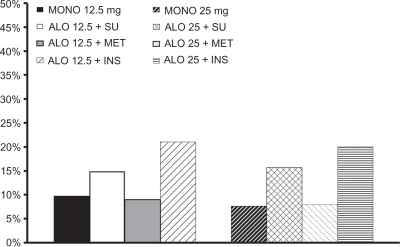
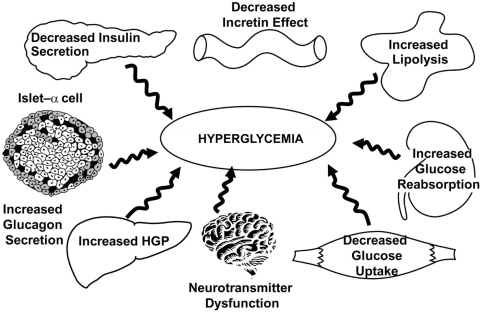
Figure 10 The ominous octet: pathophysiologic abnormalities in type 2 diabetes mellitus.Citation7