Figures & data
Table 1 Patient characteristics at the start of therapy
Table 2 Therapy of arterial hypertension before and during observation
Figure 1 Reduction in blood pressure (BP) after 6 months as a function of the systolic A) and diastolic B) initial blood pressure value.
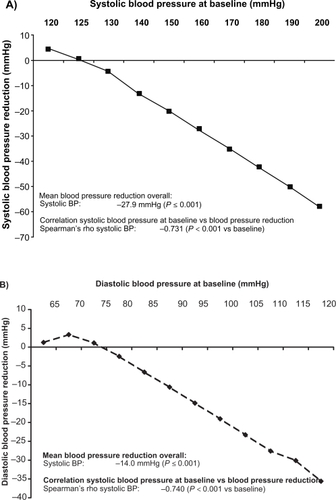
Figure 2 Normalization of blood pressure (BP) after 6 months in total and as a function of the systolic A) and diastolic B) initial blood pressure value.
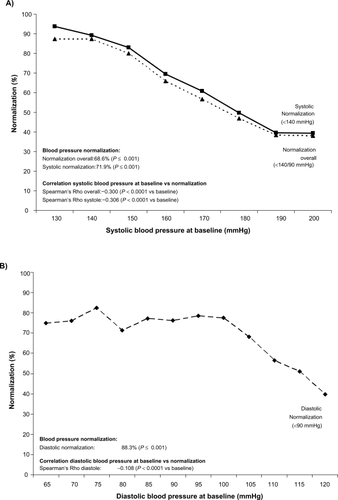
Figure 3 Reduction in systolic blood pressure (BP) (mmHg) as a function of defined patient characteristics/previous medication. Use of the Kruskal Wallis Test for differences within groups. Significant P-values for pairwise comparisons are indicated; other comparisons are not significant. Age: Lower tercile (LT) up to 58 years, middle tercile (MT) > 58 to 69 years, upper tercile (UT) > 69 years; body mass index (BMI): LT up to 25 kg/m2, MT > 25 to 30 kg/m2, UT > 30 kg/m2; duration of hypertension: LT up to 5 years, MT > 5 to 10 years, UT > 10 years.
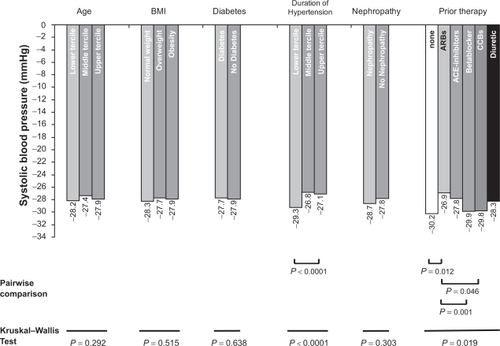
Table 3 Number of patients with adverse events (AE) without causal relationship or adverse drug reactions (ADR) in the observation period
Table 4 Adverse drug reactions (ADR) coded according to MedDRA® Version 10.0
Figure 4 Systolic normalization (<140 mmHg) according to systolic initial blood pressure value in the studies RAPiHD severeCitation10 and RAPiHD moderateCitation24 (after Franklin)Citation18. RAPiHD severe was a 7-week trial in patients with severe hypertension (seated DBP [SeDBP] ≥ 110 mmHg). Eligible patients entered a 7-day single-blind placebo lead-in period. Patients with SeDBP ≥ 110 mmHg at 2 consecutive visits during the lead-in period were randomized in a 2:1 ratio to irbesartan/HCTZ 150/12.5 mg fixed-dose combination therapy force-titrated to 300/25 mg after week 1 or irbesartan 150 mg monotherapy force-titrated to 300 mg after week 1. RAPiHD moderate was a 12-week trial in patients with moderate hypertension (seated SBP [SeSBP] 160 180 mmHg or SeDBP 100 110 mmHg). Eligible patients entered a 21-day single-blind placebo wash-out period before randomization in a 3:1:1 ratio to irbesartan/HCTZ 150/12.5 mg fixed-dose combination therapy force-titrated to 300/25 mg after week 2, irbesartan 150 mg monotherapy force-titrated to 300 mg after week 2, or HCTZ 12.5 mg monotherapy force-titrated to 25 mg after week 2.
![Figure 4 Systolic normalization (<140 mmHg) according to systolic initial blood pressure value in the studies RAPiHD severeCitation10 and RAPiHD moderateCitation24 (after Franklin)Citation18. RAPiHD severe was a 7-week trial in patients with severe hypertension (seated DBP [SeDBP] ≥ 110 mmHg). Eligible patients entered a 7-day single-blind placebo lead-in period. Patients with SeDBP ≥ 110 mmHg at 2 consecutive visits during the lead-in period were randomized in a 2:1 ratio to irbesartan/HCTZ 150/12.5 mg fixed-dose combination therapy force-titrated to 300/25 mg after week 1 or irbesartan 150 mg monotherapy force-titrated to 300 mg after week 1. RAPiHD moderate was a 12-week trial in patients with moderate hypertension (seated SBP [SeSBP] 160 180 mmHg or SeDBP 100 110 mmHg). Eligible patients entered a 21-day single-blind placebo wash-out period before randomization in a 3:1:1 ratio to irbesartan/HCTZ 150/12.5 mg fixed-dose combination therapy force-titrated to 300/25 mg after week 2, irbesartan 150 mg monotherapy force-titrated to 300 mg after week 2, or HCTZ 12.5 mg monotherapy force-titrated to 25 mg after week 2.](/cms/asset/20521a7c-0a8c-456c-9252-7a07e0a2baaf/dvhr_a_7756_f0004_c.jpg)