Figures & data
Table 1. Demographic parameters of the included patients before propensity score matching.
Table 2. Clinical and past medical history of the included patients before propensity score matching.
Table 3. Distribution of patients, expressed as numbers and percentages, in the three cohorts according to the year of inclusion and by line of treatment.
Table 4. Distribution of concomitant endocrine therapy (presented as numbers and percentages) in palbociclib, abemaciclib and ribociclib cohorts stratified by menopausal status.
CDK4/6 inhibitors prescribed (A) at the index date; (B) by 3 months among patients with at least 3 months of treatment; (C) by 6 months among patients with at least 6 months of treatment.
*Label-recommended daily dose.
NI: Not issuable due to privacy (≤3 patients).
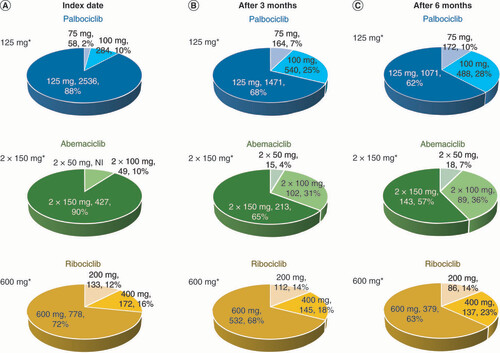
Table 5. Demographic parameters of the included patients after propensity score matching.
Table 6. Clinical and past medical history of the included patients after propensity score matching.
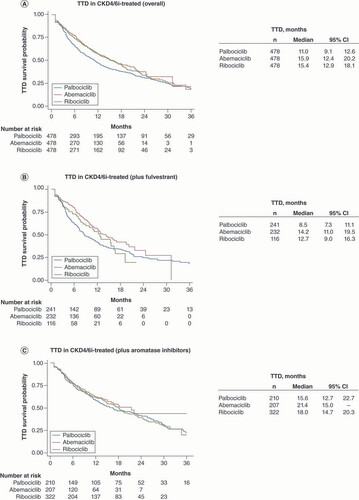
(A) Total patients receiving combined therapy with CKD4/6 and endocrine therapy, (B) patients receiving combined therapy with CKD4/6 plus fulvestrant; (C) patients receiving combined therapy with CKD4/6 plus aromatase inhibitors.
CKD4/6i: CDK4/6 inhibitor; TTD: Time to treatment discontinuation.
Data sharing statement
All data used for the current study are available upon reasonable request to CliCon S.R.L.
