Figures & data
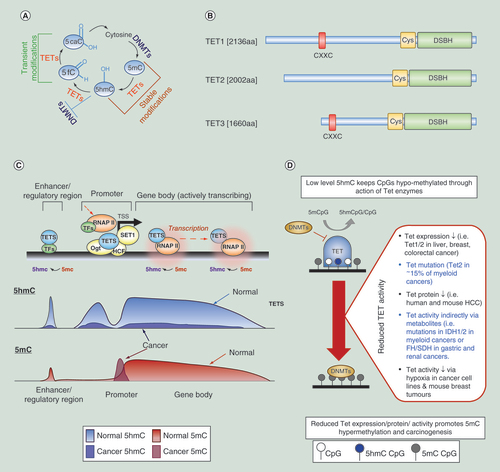
(A) Overview of the DNA methylation/active demethylation cycle. (B) Schematic of the structures of the human TET enzymes. CXXC: domain which can bind directly to unmodified CpG dinucleotides, Cys: cysteine-rich domain, DSBH: double-stranded β-helix domain including binding sites for Fe(II) and 2-OG cofactors. (C) Activation of gene expression can occur through the active demethylation of regions around the transcription start sites (loss of 5mC through a 5hmC intermediate) with elevated promoter proximal and genic 5hmC levels allowing the binding and elongation of the RNAP-II complex, probably in concert with histone modification changes (not shown). Conversion of 5mC into 5hmC (and further derivatives) is mediated by the TET proteins (blue). TET proteins have been shown to interact with OGT as well as HCF1, component of the H3K4 methyltransferase SET1/COMPASS complex, resulting in altered chromatin environments (OGT, HCF1&SET1 complex = Yellow). Thymine DNA glycosylase, TDG, (not shown) is proposed to complete this promoter specific demethylation through base excision repair while regions lacking TDG lead to the accumulation of 5hmC (such as gene bodies and enhancers), perhaps by tracking the RNA Pol II complex in the case of gene bodies. Typical 5hmC (blue) and 5mC (red) profiles at an actively transcribing gene are shown below with patterns observed in cancer shown as an overlay. (D) Review of methods by which Tet enzyme levels or activity may be altered and how these may be related to perturbed epigenetic landscapes in cancer.
5hmC: 5-hydroxymethylcytosine; HCF1: Host Cell Factor 1; OGT: O-GlcNAc transferase.
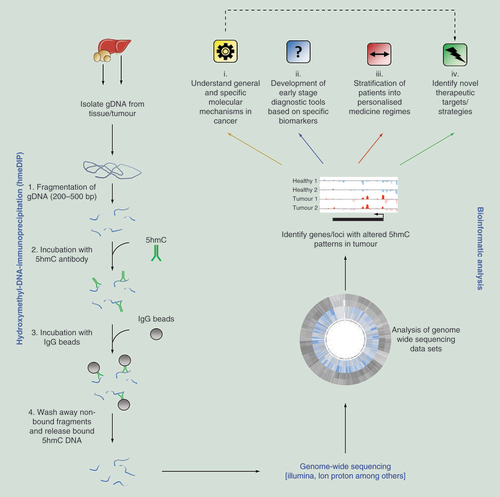
Genomic DNA (gDNA) is isolated from the host organ/tumor and fragmented to a desired size range. Following enrichment of 5hmC marked DNA fragments by hydroxymethyl-DNA-immunoprecipitation (hmeDIP), sequencing libraries can be prepared and genome-wide sequencing carried out. Following bioinformatic processing, regions of differential 5hmC modification can be identified which can lead to: (i) increased understanding of molecular mechanisms, (ii) identification of diagnostic, (iii) or cancer specific (patient stratification) biomarkers and (iv) identification of novel therapeutic targets.
5hmC: 5-hydroxymethylcytosine.
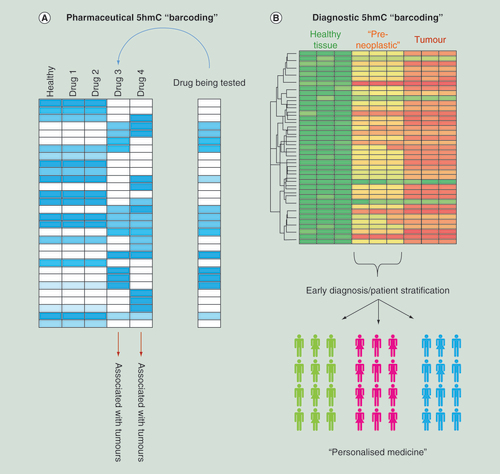
(A) Schematic examples for how epigenetic ‘barcoding’ based on genome-wide 5hmC patterns can aid in the stratification of novel drug compounds depending on potential carcinogenic outcome. (B) Schematic for how 5hmC ‘barcoding’ could be applied to identify a series of epigenetic changes which occur prior to the observation of a tumor. In doing so it may be possible to stratify patients into cohorts based on their specific cancer subtype which would aid in downstream personal medicine regimes.
5hmC: 5-hydroxymethylcytosine.
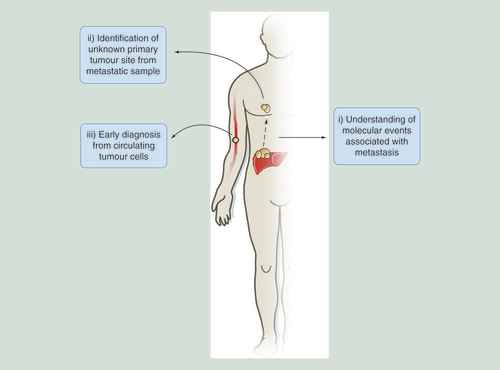
Examples for how 5hmC-based studies can aid in probing of molecular events that occur in metastasis, resulting in the identification of primary tumor sites distinct from metastatic samples or be adapted for novel early stage diagnostic tools through the study of circulating tumor cells in the bloodstream.
5hmC: 5-hydroxymethylcytosine.
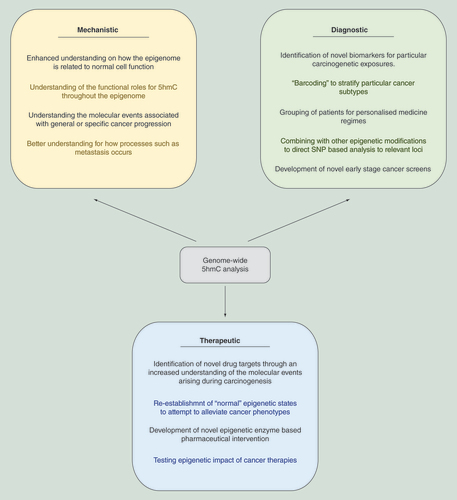
5hmC-based analysis can aid both the understanding of the underlying mechanisms associated with cancer progression, identify novel diagnostic tools and result in the development of new therapeutic strategies for cancer treatment.
5hmC: 5-hydroxymethylcytosine.