Figures & data
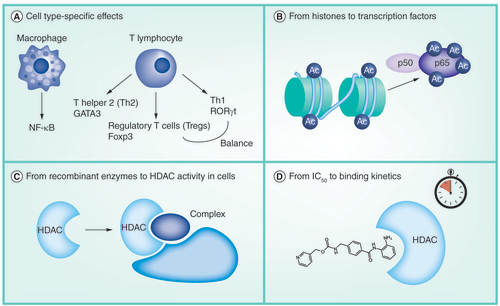
(A) Zn2+-dependent HDACs. (B) NAD+-dependent HDACs.
HDAC: Histone deacetylase.
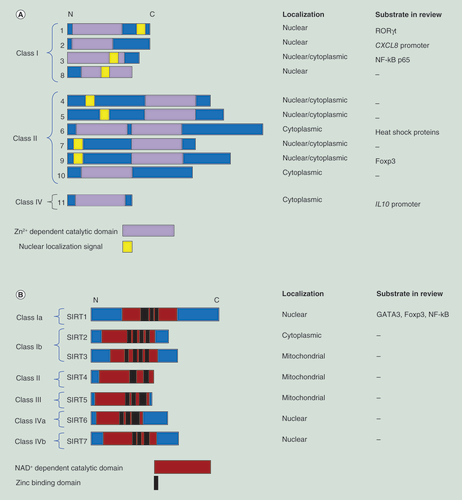
(A) Macrophages and various T lymphocytes have been implicated in inflammatory airway diseases such as asthma and chronic obstructive pulmonary disease. These cell types depend on specific transcription factors for their differentiation and functionality. Histone deacetylases (HDACs) can affect the acetylation of these transcription factors, which influences, for example, the transcription factor stability or its DNA binding capability. HDAC inhibitors (HDACi) can influence these biological processes and thereby influence inflammatory responses by these cell types. (B) Effects of HDACi on histone acetylation are often used as a read-out indicative of the effects of these compounds in cells. However, histone acetylation is not sufficient to (fully) explain the effects of HDACi on biological processes such as gene expression. Effects of HDACi on transcription factors and their acetylation status may also be particularly important in explaining these effects. (C) Interestingly, selectivity profiles of HDACi are commonly elucidated by testing for inhibition of recombinant HDACs. Importantly, however, in cells HDACs are known to be present in multiprotein complexes that influence their activity. Importantly, recently, HDACi have been reported to have different specificities for such complexes, which adds another layer of complexity to the selectivity profile. (D) Different classes of HDACi have been reported to have different binding kinetics with the HDACs. Benzamine-based inhibitors have slow binding kinetics, while hydroxamate-based inhibitors have fast binding kinetics. This has been demonstrated to translate into biological consequences.