Future Cardiology
Volume 20, 2024 - Issue 2
Open access
729
Views
0
CrossRef citations to date
0
Altmetric
Short Communication
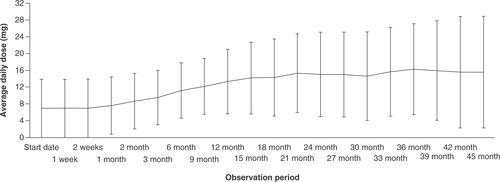
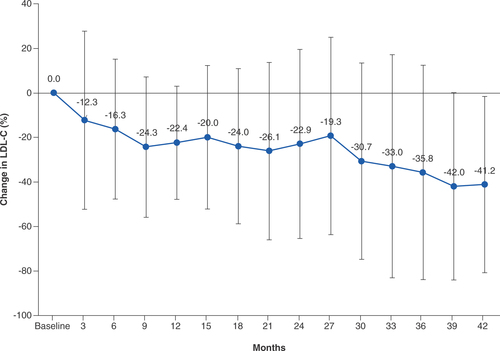
Real-world safety and efficacy of lomitapide in homozygous familial hypercholesterolemia: interim report of special-use survey in Japan
Mariko Harada-Shiba1 Cardiovascular Center, Osaka Medical & Pharmaceutical University, Osaka, 569-8686, JapanCorrespondence[email protected]
 https://orcid.org/0000-0002-7600-2059
https://orcid.org/0000-0002-7600-2059
Shigenori Haruna2 Recordati Rare Diseases Japan K.K., Tokyo, 102-0082, Japan
& Noriaki Kogawa2 Recordati Rare Diseases Japan K.K., Tokyo, 102-0082, Japan
Pages 67-80
|
Received 13 Nov 2023, Accepted 05 Feb 2024, Published online: 29 Feb 2024
Related research
People also read lists articles that other readers of this article have read.
Recommended articles lists articles that we recommend and is powered by our AI driven recommendation engine.
Cited by lists all citing articles based on Crossref citations.
Articles with the Crossref icon will open in a new tab.