Figures & data
Mirvetuximab soravtansine binds with high affinity to folate receptor-α expressed on the tumor cell surface, prompting internalization of the antibody–drug conjugate/receptor complex via antigen-mediated endocytosis. Lysosomal processing releases active DM4 catabolites – these maytansinoid derivatives inhibit tubulin polymerization and microtubule assembly, inducing potent antimitotic effects that result in cell-cycle arrest and apoptosis. The active metabolites may also diffuse into neighboring cells and induce further cell death (termed ‘bystander killing’).
Reproduced with permission from [Citation30].
![Figure 1. Mirvetuximab soravtansine mechanism of action.Mirvetuximab soravtansine binds with high affinity to folate receptor-α expressed on the tumor cell surface, prompting internalization of the antibody–drug conjugate/receptor complex via antigen-mediated endocytosis. Lysosomal processing releases active DM4 catabolites – these maytansinoid derivatives inhibit tubulin polymerization and microtubule assembly, inducing potent antimitotic effects that result in cell-cycle arrest and apoptosis. The active metabolites may also diffuse into neighboring cells and induce further cell death (termed ‘bystander killing’).Reproduced with permission from [Citation30].](/cms/asset/f294214a-5df8-4578-9bc1-ade23413f56d/ifon_a_12331908_f0001.jpg)
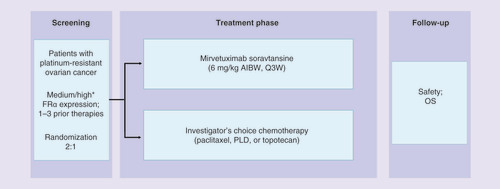
AIBW: Adjusted ideal body weight; OS: Overall survival; PLD: Pegylated liposomal doxorubicin.
Countries with active sites are shown in green, those with pending sites are shown in yellow (as of January 2018).