Figures & data
GERSC: Granisetron extended-release subcutaneous; HEC: Highly emetogenic chemotherapy; MEC: Moderately emetogenic chemotherapy.
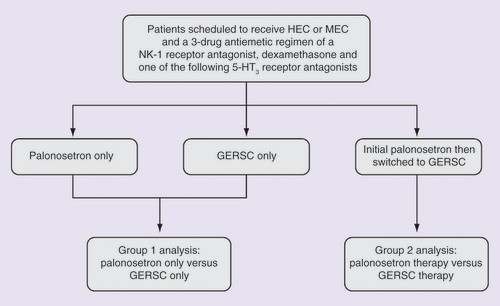
Table 1. Patient demographics and baseline characteristics.
Table 2. Group 1 analysis: number of chemotherapy cycles and hydration rate.
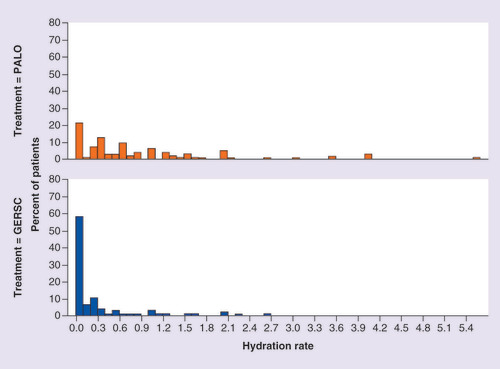
Distribution of hydration rate by chemotherapy cycle and by antiemetic regimen.
GERSC: Granisetron extended-release subcutaneous; PALO: Palonosetron.
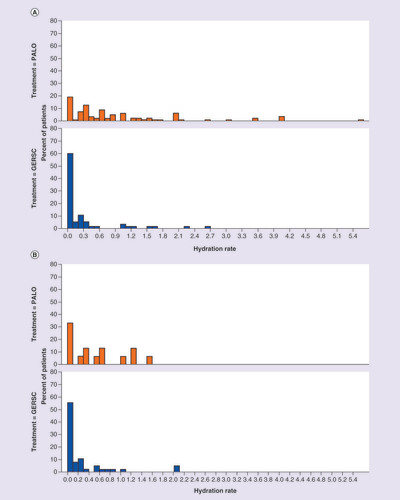
Distribution of hydration rates by chemotherapy cycle and by antiemetic regimen for patients receiving (A) highly emetogenic chemotherapy or (B) moderately emetogenic chemotherapy.
GERSC: Granisetron extended-release subcutaneous; PALO: Palonosetron.