Figures & data
Table 1.
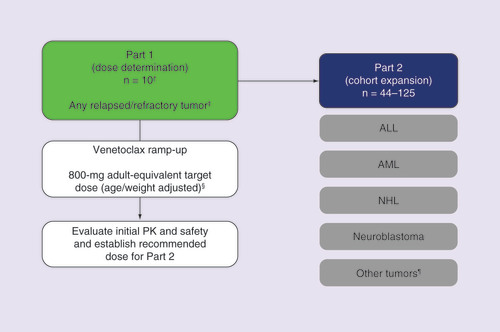
Mechanism-based selection of pediatric tumor types.
Table 2.
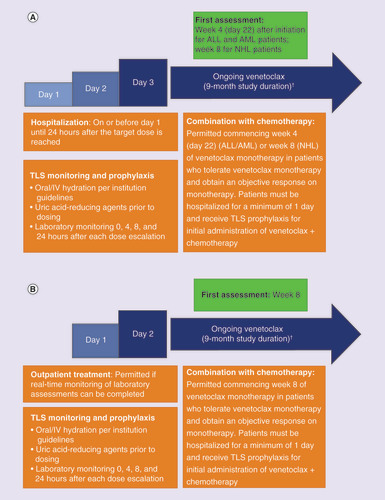
Venetoclax dose ramp-up in patients with hematologic malignancies or lymphomas.
Table 3.
Venetoclax dose ramp-up in patients with solid tumors.
Table 4.
Allowed chemotherapy regimens in combination with venetoclax for each tumor type.
Table 5.
Key inclusion and exclusion criteria.
Table 6.
Efficacy assessment criteria for each tumor type.
![Figure 1. Mechanism of action of venetoclax.MOMP: Mitochondrial outer membrane permeabilization.Adapted from Cancer Discovery, 2016, 6(10), 1106–1117, Konopleva M, Pollyea DA, Potluri J et al. Efficacy and biological correlates of response in a Phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia, with permission from AACR [Citation6].](/cms/asset/c2a1b07b-2943-48e2-8fe3-169448780da6/ifon_a_12331960_f0001.jpg)