Figures & data
Table 1. Patient demographics and clinical status; cancer and chemotherapy-induced neutropenia/febrile neutropenia history and management.
Table 2. Zarzio® prophylaxis patterns.
†Secondary prophylaxis started in cycle 2 or later despite no CIN/FN in prior cycle.
C: Correctly-prophylacted; CIN: Chemotherapy-induced neutropenia; DLBCL: Diffuse large B-cell lymphoma; FN: Febrile neutropenia; MM: Multiple myeloma; O: Over-prophylacted; U: Under-prophylacted.
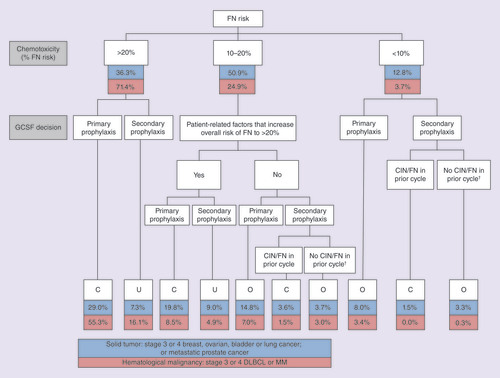
Solid tumor: stage 3 or 4 breast, ovarian, bladder or lung cancer; or metastatic prostate cancer. Hematological malignancy: stage 3 or 4 diffuse large B-cell lymphoma or multiple myeloma. CIN grades 1–4, grades 3 or 4, grade 4 and FN not mutually exclusive.
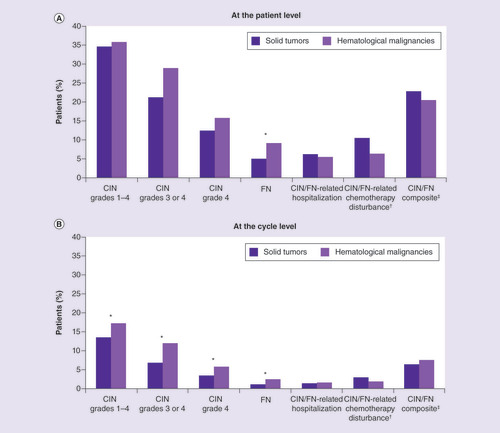
†Chemotherapy disturbance includes dose reduction, discontinuation or delay. Measured with one-cycle lag.
‡Includes any occurrence of CIN grade 4, FN, CIN/FN-related hospitalization and/or CIN/FN-related chemotherapy disturbance.
* Difference between solid tumor and hematological malignancy cohorts significant at p < 0.05.
CIN: Chemotherapy-induced neutropenia; FN: Febrile neutropenia.
Supplemental Table 1
Download MS Word (49.1 KB)Data sharing statement
The authors certify that this manuscript reports the secondary analysis of clinical trial data that have been shared with them, and that the use of this shared data is in accordance with the terms (if any) agreed upon their receipt. The source of this data is: NCT01459653.
