Figures & data
Table 1. Inclusion and exclusion criteria based on the Population, Intervention, Comparator, Outcomes Study Designs framework.
PRISMA: Preferred reporting items for systematic reviews and meta-analyses; RCT: Randomized controlled trial; RWD: Real-world data; TKI: Tyrosine kinase inhibitor.
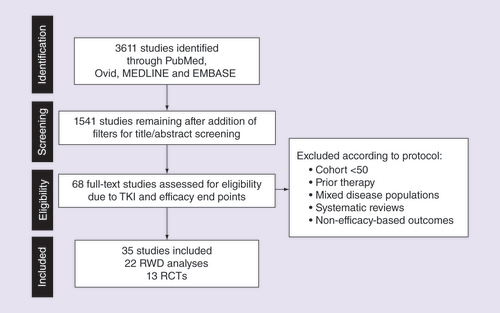
Table 2. Number of studies, treatment arms and patients by end point and group.
Table 3. Number of studies by publication year and study type for median progression-free survival.
*1 or 2 denotes TKI treatment group, 0 denotes non-TKI treatment group.
mPFS: Median progression-free survival; RCT-control: Matching control, non-TKI treatments in randomized controlled trial; RCT-TKI: TKI as a single agent in the first-line setting in randomized controlled trials; RWD-TKI: TKI as a single agent in the first-line setting in real-world data study; TKI: Tyrosine kinase inhibitor; Tx: Treatment group.
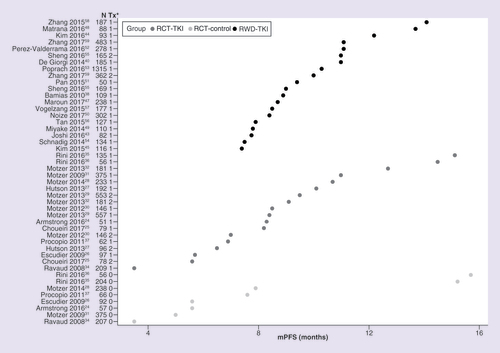
*1 or 2 denotes TKI treatment group, 0 denotes non-TKI treatment group.
**mOS data were not mature at the time of publication and were extracted from a later analysis [Citation33].
mOS: Median overall survival; RCT-control: Matching control, non-TKI treatments in randomized controlled trial; RCT-TKI: TKI agent in randomized controlled trial; RWD-TKI: TKI as a single agent in the first-line setting in real-world data study; TKI: Tyrosine kinase inhibitor; Tx: Treatment group.
![Figure 3. Observed median overall survival data.*1 or 2 denotes TKI treatment group, 0 denotes non-TKI treatment group.**mOS data were not mature at the time of publication and were extracted from a later analysis [Citation33].mOS: Median overall survival; RCT-control: Matching control, non-TKI treatments in randomized controlled trial; RCT-TKI: TKI agent in randomized controlled trial; RWD-TKI: TKI as a single agent in the first-line setting in real-world data study; TKI: Tyrosine kinase inhibitor; Tx: Treatment group.](/cms/asset/c43e6e30-110b-4ddd-b70a-3559fc36ad85/ifon_a_12332445_f0003.jpg)
*1 or 2 denotes TKI treatment group, 0 denotes non-TKI treatment group.
ORR: Objective response rate; RCT-control: Matching control, non-TKI treatments in randomized controlled trial; RCT-TKI: TKI agent in randomized controlled trial; RWD-TKI: TKI as a single agent in the first-line setting in real-world data study; TKI: Tyrosine kinase inhibitor; Tx: Treatment group.
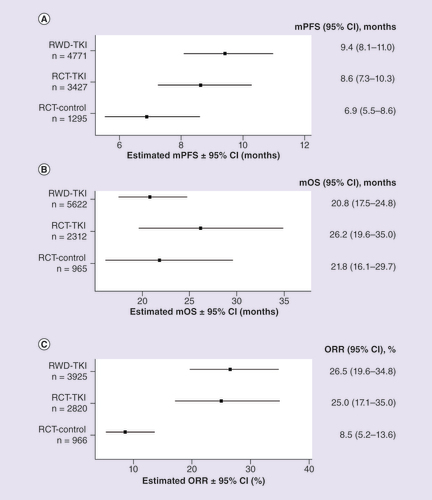
Forest plot of (A) mPFS, (B) mOS and (C) ORR for patients with mRCC treated with first-line TKIs.
mOS: Median overall survival; mPFS: Median progression-free survival; mRCC: Metastatic renal cell carcinoma; ORR: Objective response rate; RCT: Randomized controlled trial; RCT-control: Matching control, non-TKI in randomized controlled trial; RCT-TKI: TKI agent in randomized controlled trial; RWD: Real-world data; RWD-TKI: TKI agent in RWD study; TKI: Tyrosine kinase inhibitor.
Data sharing statement
Upon request, and subject to certain criteria, conditions and exceptions (https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices for indications that have been approved in the USA and/or the EU or; in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requesters must enter into a data access agreement with Pfizer.
