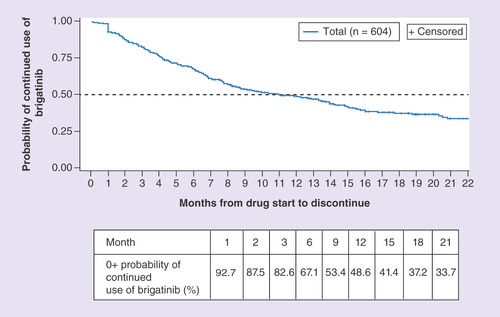
Figures & data
Table 1. Baseline characteristics of patients in the brigatinib early access program.
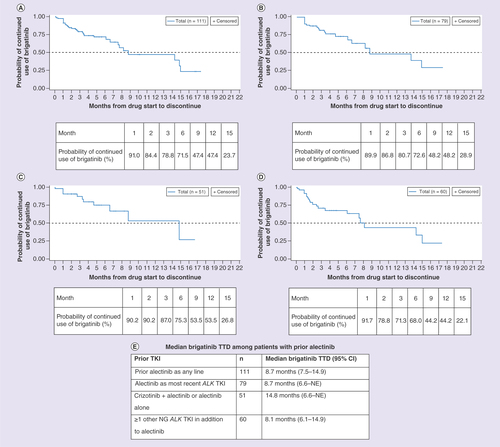
(A) Patients with prior alectinib as any line. (B) Patients with alectinib as most recent ALK TKI. (C) Patients with crizotinib + alectinib or alectinib alone. (D) Patients with ≥1 other NG ALK TKI in addition to alectinib. (E) Median brigatinib TTD among patients with prior alectinib.
NG: Next-generation; TKI: Tyrosine kinase inhibitor; TTD: Time-to-treatment discontinuation.
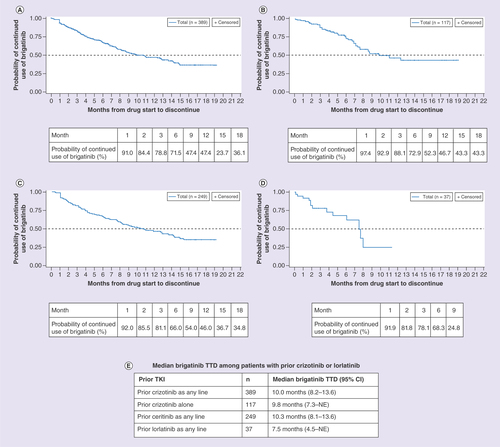
(A) Patients with prior crizotinib as any line. (B) Patients with prior crizotinib alone. (C) Patients with prior ceritinib as any line. (D) Patients with prior lorlatinib as any line. (E) Median brigatinib TTD among patients with prior crizotinib or lorlatinib.
NE: Not evaluable; TKI: Tyrosine kinase inhibitor; TTD: Time-to-treatment discontinuation.
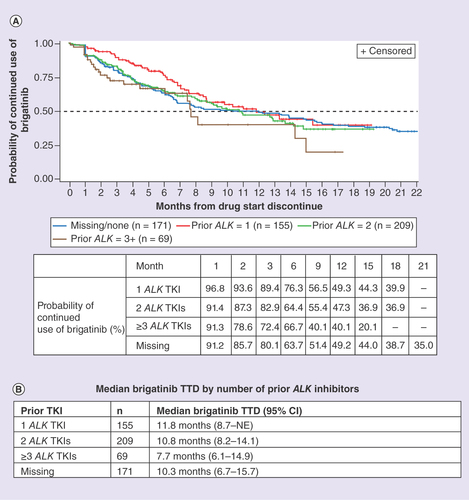
(A) Patients with one to three prior ALK TKIs. (B) Median brigatinib TTD by number of prior ALK inhibitors.
NE: Not evaluable; TKI: Tyrosine kinase inhibitor; TTD: Time-to-treatment discontinuation.