Figures & data
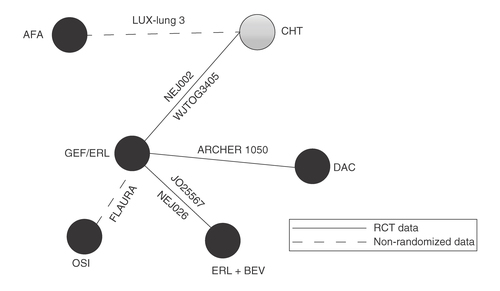
Non randomized data was defined as any post hoc subgroup data that broke randomization; LUX-Lung 3 and FLAURA reported post hoc Japanese subgroup results, however, the randomization was not stratified by Japanese ethnicity.
AFA: Afatinib; BEV: Bevacizumab; CICIS: Cisplatin; DAC: Dacomitinib; ERL: Erlotinib; GEF: Gefitinib; OSI: Osimertinib.