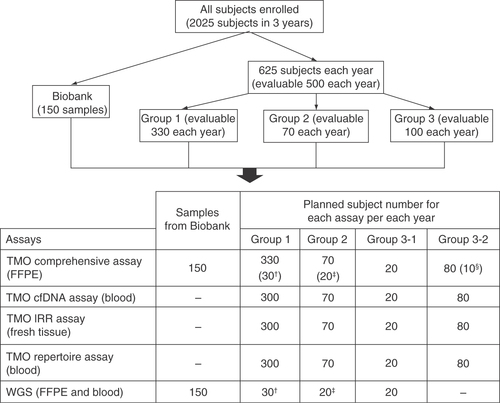
Figures & data
TMO comprehensive assay, Oncomine Comprehensive Assay v3; TMO cfDNA assay, Oncomine Breast cfDNA Assay; TMO IRR assay, Oncomine Immune Response Research Assay; TMO repertoire assay, Ion AmpliSeq Immune Repertoire Assay Plus-TCR beta.
†Subjects with breast cancer recurrence at screening.
‡Subjects who do not achieve pathological complete response (non-pCR), have breast cancer recurrence, and with paired tumor formalin-fixed paraffin-embedded tissues available.
§Subjects who are currently receiving the first-line treatment for mBC and have PD within 3 months after initiating the first-line treatment for mBC (subjects with rapid PD).
BC: Breast cancer; mBC: Metastatic breast cancer; PD: Progressive disease; TMO: Thermo Fisher Oncomine; WGS: Whole-genome sequencing.