Figures & data
Table 1. Patient characteristics.
Table 2. Efficacy of docetaxel plus nintedanib post-immune checkpoint inhibitor.
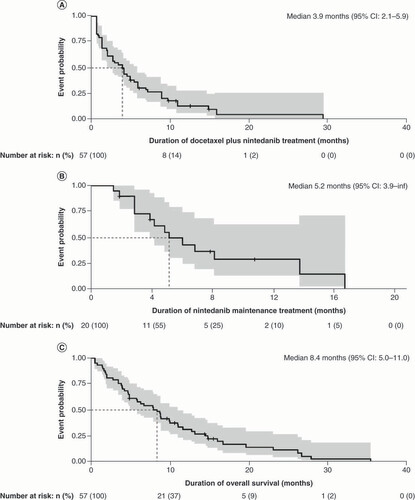
Kaplan-Meier curves illustrating the third-line treatment outcomes with docetaxel plus nintedanib following first-line chemotherapy and second-line immune checkpoint inhibitors, representing (A) treatment duration with docetaxel plus nintedanib (n = 57), (B) maintenance treatment with nintedanib (n = 20) and (C) overall survival following third-line docetaxel plus nintedanib treatment (n = 57).
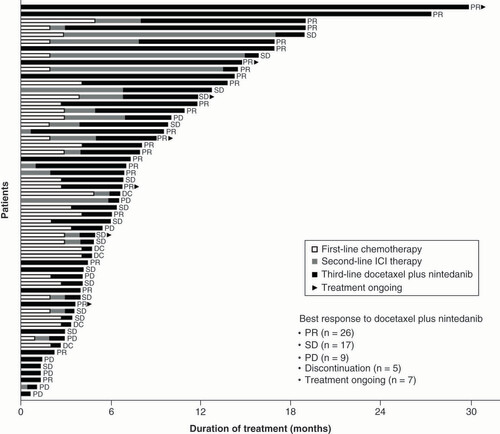
Data presented for 57 patients who received third-line docetaxel plus nintedanib. Treatment duration for first-line therapy was not available for 23 patients. Treatment duration for second-line therapy was not available for 33 patients.
DC: Discontinuation; ICI: Immune checkpoint inhibitor; PD: Progressive disease; PR: Partial response; SD: Stable disease.
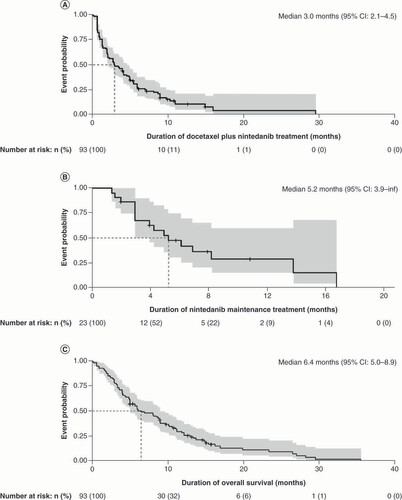
Kaplan-Meier curves illustrating the overall treatment outcomes with docetaxel plus nintedanib across all treatment lines included in the analysis, representing (A) duration of treatment with docetaxel plus nintedanib (n = 93), (B) maintenance treatment with nintedanib (n = 23) and (C) overall survival following treatment with docetaxel plus nintedanib (n = 93).