Figures & data
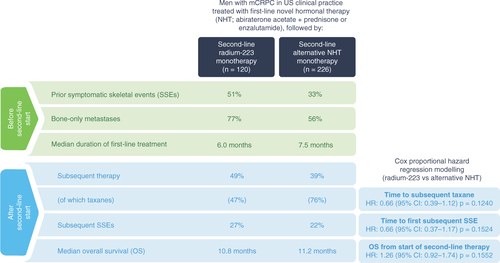
mCRPC: Metastatic castration-resistant prostate cancer; NHT: Novel hormonal therapy.
Table 1. Patient demographics and disease characteristics.
Table 2. Subsequent life-prolonging therapy after second-line therapy.
†Patients were counted once for each treatment type, regardless of the number of lines received; patients who received more than one treatment type were counted in each category.
‡The denominator is the number of patients in each cohort who received any subsequent therapy (radium-223 n = 59; alternative NHT n = 88).
§In the radium-223 cohort, one patient received subsequent radium-223 monotherapy; in the alternative NHT cohort, one patient received subsequent pembrolizumab monotherapy, and one patient received subsequent sipuleucel-T monotherapy.
NHT: Novel hormonal therapy.
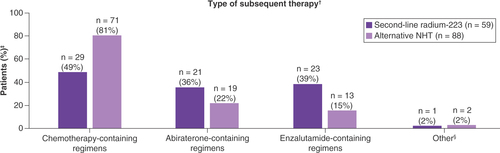
Table 3. Multivariable Cox proportional hazards regression model for time to first use of subsequent taxane therapyTable Footnote†, Table Footnote‡.
Table 4. Multivariable cox proportional hazards regression model for symptomatic skeletal event occurrenceTable Footnote†, Table Footnote‡.
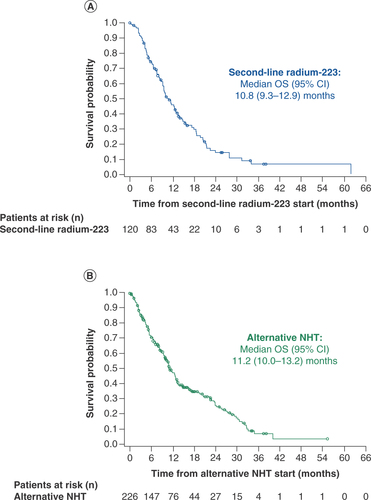
(A) Radium-223 cohort. (B) Alternative NHT cohort.
NHT: Novel hormonal therapy; OS: Overall survival.