Figures & data
Table 1. Final attributes and levels included in the discrete choice experiment.
CV: Cardiovascular; TKI: Tyrosine kinase inhibitor.
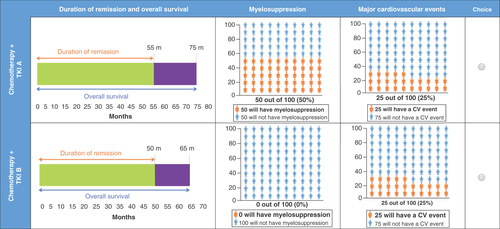
Table 2. Patient demographics and baseline characteristics in the main survey.
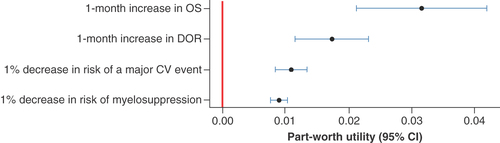
Discrete choice experiment preference data collected from the main survey were analyzed using a linear multinomial logit model. Preferences for changes in the attributes are expressed as part-worth utilities. Markers indicate maximum likelihood estimates, and the bars indicate 95% CI.
CV: Cardiovascular; DOR: Duration of remission; OS: Overall survival.
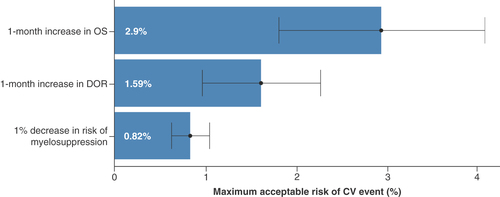
CV: Cardiovascular; DOR: Duration of remission; OS: Overall survival.
Maximum acceptable CV risk for 1 month additional OS by participant subgroups.
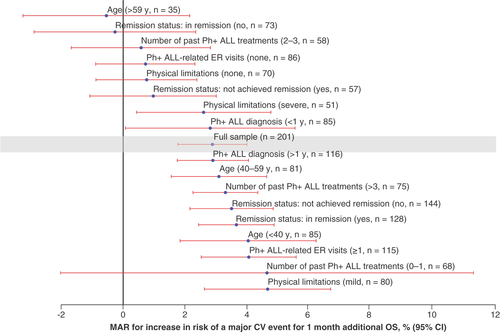
CV: Cardiovascular; ER: Emergency room; MAR: Maximum acceptable risk; OS: Overall survival; Ph+ ALL: Philadelphia chromosome-positive acute lymphoblastic leukemia; y: Year.