Figures & data
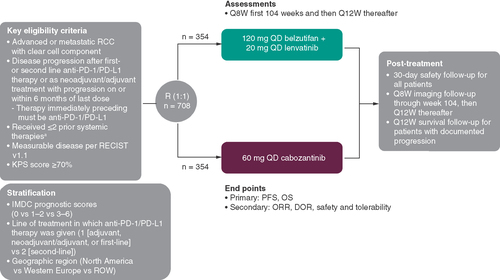
aIncluding 1 anti-PD-1/PD-L1-containing adjuvant or neoadjuvant/adjuvant regimens with progression on or within 6months from the last dose of that regimen OR 1 or 2 regimens for locoregional/advanced disease.
DOR: Duration-of-response; IMDC: International Metastatic Renal Cell Carcinoma Database Consortium; KPS: Karnofsky Performance Status Scale; ORR: Objective response rate; OS: Overall survival; PD-1: Programmed death 1; PD-L1: Programmed death ligand 1; PFS: Progression-free survival; Q8W: Every 8 weeks; Q12W: Every 12 weeks; QD: Once daily; R: Randomization; RCC: Renal cell carcinoma; RECIST 1.1: Response Evaluation Criteria in Solid Tumors, version 1.1; ROW: Rest of world.