Future Oncology
Volume 19, 2023 - Issue 7
Open access
1,123
Views
3
CrossRef citations to date
0
Altmetric
Clinical Trial Protocol
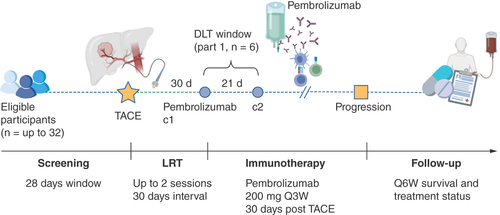
PETAL protocol: a phase Ib study of pembrolizumab after transarterial chemoembolization in hepatocellular carcinoma
Petros Fessas1 Department of Surgery & Cancer, Imperial College London,Hammersmith Hospital,
Du Cane Road, W120HS,
London,
UKView further author information
, Bernhard Scheiner1 Department of Surgery & Cancer, Imperial College London,Hammersmith Hospital,
Du Cane Road, W120HS,
London,
UK;5 Liver Cancer (HCC) Study Group,Division of Gastroenterology & Hepatology, Department of Internal Medicine III, Medical University of Vienna,
Vienna,
AustriaView further author information
, Antonio D’Alessio1 Department of Surgery & Cancer, Imperial College London,Hammersmith Hospital,
Du Cane Road, W120HS,
London,
UK;2 Department of Biomedical Sciences,Humanitas University,
Via Rita Levi Montalcini 4,
20072,
Pieve Emanuele,
Milan,
ItalyView further author information
, Claudia A M Fulgenzi1 Department of Surgery & Cancer, Imperial College London,Hammersmith Hospital,
Du Cane Road, W120HS,
London,
UK;3 Department of Medical Oncology,University Campus Bio-Medico of Rome,
Rome, ItalyView further author information
, James Korolewicz1 Department of Surgery & Cancer, Imperial College London,Hammersmith Hospital,
Du Cane Road, W120HS,
London,
UKView further author information
, Caroline Ward1 Department of Surgery & Cancer, Imperial College London,Hammersmith Hospital,
Du Cane Road, W120HS,
London,
UKView further author information
, Paul Tait4 Department of Radiology, Imperial College NHS Trust,Hammersmith Hospital,
Du Cane Road,
W120HS,
London,
UKView further author information
, Robert Thomas4 Department of Radiology, Imperial College NHS Trust,Hammersmith Hospital,
Du Cane Road,
W120HS,
London,
UKView further author information
, Alessio Cortellini1 Department of Surgery & Cancer, Imperial College London,Hammersmith Hospital,
Du Cane Road, W120HS,
London,
UKView further author information
, Rohini Sharma1 Department of Surgery & Cancer, Imperial College London,Hammersmith Hospital,
Du Cane Road, W120HS,
London,
UKView further author information
& David J Pinato1 Department of Surgery & Cancer, Imperial College London,Hammersmith Hospital,
Du Cane Road, W120HS,
London,
UK;6 Division of Oncology, Department of Translational Medicine,University of Piemonte Orientale,
Novara,
ItalyCorrespondence[email protected]
View further author information
show allView further author information
Pages 499-507
|
Received 15 Sep 2022, Accepted 16 Feb 2023, Published online: 25 Apr 2023
Related research
People also read lists articles that other readers of this article have read.
Recommended articles lists articles that we recommend and is powered by our AI driven recommendation engine.
Cited by lists all citing articles based on Crossref citations.
Articles with the Crossref icon will open in a new tab.